-
PDF
- Split View
-
Views
-
Cite
Cite
Gregory S. Simpson, Sunando R. Mahapatra, James Evans, Incidental complete excision of appendiceal gastric cancer metastasis, Journal of Surgical Case Reports, Volume 2013, Issue 10, October 2013, rjt080, https://doi.org/10.1093/jscr/rjt080
Close - Share Icon Share
Abstract
Gastric cancer is a common malignancy with high recurrence rates following surgical resection. A common site of disease recurrence is the peritoneum. We report the case of a 73-year-old female who had previously undergone a total gastrectomy for gastric cancer who presented acutely with features classical of acute appendicitis and underwent open appendectomy. Histological analysis showed metastasis of gastric cancer with clear resection margins. The patient recovered fully and has remained disease-free for 14 months following presentation. Peritoneal metastasis is associated with difficulty in treatment and poor prognosis. The unplanned excision of this patient’s peritoneal metastasis has yielded a favourable clinical outcome in a difficult clinical situation.
INTRODUCTION
Gastric cancer is the fourth most prevalent malignancy worldwide, and is responsible for the third greatest number of deaths due to cancer [1]. The spread of gastric cancer can occur as a consequence of lymphatic dissemination or by direct invasion of adjacent structures. Spread may occur to the liver, lungs, peritoneum, bone or brain. Recurrence of gastric cancer is common, with the peritoneum a frequently involved site, with rates of peritoneal recurrence between 17 and 46% [2–4]. Recurrence commonly occurs early following initial surgical resection, often within 1 year [5]. Multiple independent risk factors for peritoneal recurrence have been described in the literature including gastric serosal infiltration, lymph node involvement, tumour size and diffuse-mixed histological type [4, 6].
CASE REPORT
We report an interesting case of metastatic gastric cancer involving a 73 year old initially diagnosed endoscopically with moderately differentiated gastric adenocarcinoma diffusely involving the fundus, lesser and greater curvatures. Following diagnosis, the patient underwent staging computed tomography (CT) of the chest, abdomen and pelvis and staging laparoscopy, with peritoneal lavage cytology. Both staging investigations revealed no evidence of metastatic disease in the abdominal organs, peritoneum or chest. Following diagnosis, the patient was commenced on a neoadjuvant chemotherapy regime using epirubicin, capecitabine and cisplatin; however, she was unable to tolerate the chemotherapy due to toxicity and received only one cycle, after which no further neoadjuvant chemotherapy was administered (Fig. 1).
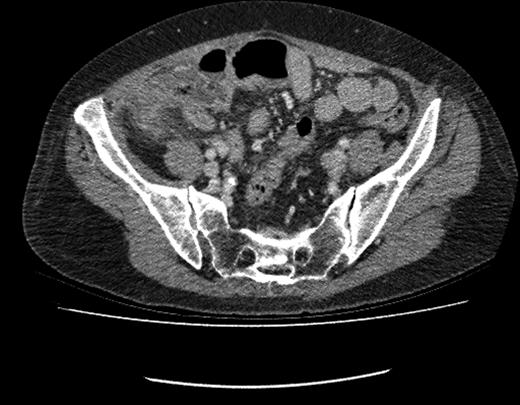
CT image showing fluid-filled appendix with peri-caecal fat stranding.
Following incomplete neoadjuvant chemotherapy, this patient underwent a total gastrectomy with Roux-en-Y reconstruction, which proceeded without complication. Histological assessment of the resected specimen staged the tumour as T3 N2, with 17 of 27 resected lymph nodes positive for disease. Margin positivity was identified at the oesophageal resection margin. The patient recovered rapidly from this operative intervention and returned home. Following surgical resection, the patient remained well.
This patient returned to hospital 13 months following surgical resection of her gastric malignancy with an acute onset of abdominal pain. The patient gave a classical history of acute appendicitis with initial peri-umbilical pain, followed by localization of the pain to the right iliac fossa. Clinical findings and biochemical markers were consistent with this clinical suspicion. Prior to surgery a contrast-CT scan was performed. CT imaging indicated a fluid-filled appendix with peri-caecal fat stranding indicative of acute appendicitis. The patient consequently underwent an open appendectomy.
Intra-operatively, typical appearances of appendicitis were identified with a gangrenous, inflamed appendix. A perforation was noted at the base of the appendix with local faecal contamination. The appendix was successfully removed, the base ligated and oversewn and a thorough washout of the pelvis and paracolic gutter performed. The patient was commenced on i.v. piperacillin and tazobactam post-operatively and returned home 3 days following the procedure with no intra-operative or post-operative complications noted.
Histological assessment of the appendix specimen revealed extensive infiltration from serosa to the muscularis propria by adenocarcinoma cells. Upon immunohistochemical analysis, cells were positive for cytokeratin 7, but negative for cytokeratin 20, CD56, synaptophysin and chromogranin, mirroring the immunohistochemical findings of the patient's original gastric cancer.
The patient remained well post-operatively and made a full recovery; 14-month post-operatively, the patient underwent CT imaging of the abdomen, which revealed no evidence of disease recurrence. The patient continues to have no evidence of disease recurrence 2 years following resection of the appendix.
DISCUSSION
Pre-operative staging of gastric cancer is most commonly undertaken by CT. Additional techniques can be employed to compliment this imaging modality and include staging laparoscopy and peritoneal lavage cytology to assess for peritoneal deposits of gastric cancer. The use of staging laparoscopy has been advocated in the literature [7], with centres identifying that it may be employed with minimal risk while yielding increased accuracy of pre-operative staging. In studies available where a discrepancy is identified between CT staging and staging laparoscopy, this generally occurs where laparoscopic assessment identifies previously unseen peritoneal deposits and therefore upstages disease [8–10]. Peritoneal lavage cytology may identify additional evidence of peritoneal spread in patients where macroscopic disease is not detected by staging laparoscopy.
Treatment options for advanced disease with peritoneal spread are limited. Cytoreductive surgical techniques have been described. Treatment protocols involving oral, systemic or intra-peritoneal administration of chemotherapeutic agents are also utilized.
Peritoneal metastasis of gastric cancer is a common pattern of disease progression, and is associated with a poor prognosis. On initial staging investigations, the patient described was not found to have metastatic disease. The high sensitivity of diagnostic laparoscopy makes the presence of the above-mentioned appendiceal metastases at the time of staging laparoscopy, peritoneal lavage cytology and CT imaging unlikely. The case presented represents a difficult clinical situation pre-operatively with limitation of the administration of optimum neoadjuvant therapy due to poor patient tolerance followed by incomplete surgical resection with positive resection margins.
The excision of metastatic deposits to the vermiform appendix in this case occurred incidentally during surgery for what was believed to be acute appendicitis based on clinical findings and CT evidence. The appendectomy performed appears to have incidentally removed the patients existing peritoneal metastases completely, and since the time of operation, the patient has no further evidence of peritoneal metastases on imaging and remains well.



