-
PDF
- Split View
-
Views
-
Cite
Cite
D Lam, KP Croome, R Hernandez-Alejandro, Pancreas-sparing duodenectomy for an obstructive adenocarcinoma of the duodenum, Journal of Surgical Case Reports, Volume 2012, Issue 8, August 2012, Page 13, https://doi.org/10.1093/jscr/2012.8.13
Close - Share Icon Share
Abstract
A duodenal adenocarcinoma arising from the junction of the second and third portion of the duodenum, which was resected by pancreas-sparing duodenectomy, is reported. The completely obstructing tumour was circumferential and measured 6.5cm x 3.5cm x 1.0 cm. There was no evidence of pancreas invasion, nor any lymph node metastasis. Pancreas-sparing duodenectomy was performed, with dissection of the pancreaticoduodenal lymph nodes. The proximal duodenum was transected just distal to the ampula of Vater and jejunum was transected just distal to the ligament of Treitz. A hand-sewn side-to-side anastomosis for the duodenojejunostomy was performed. There were no postoperative complications. Pathology reported a duodenal adenocarcinoma resected with negative margins. Pancreaticoduodenectomy is the treatment of choice for a duodenal adenocarcinoma, however, pancreas-sparing duodenectomy may be a safe alternative for duodenal tumours not involving the 2nd portion, especially in elderly patients with multiple medical comorbidities.
INTRODUCTION
Adenocarcinomas account for the majority of neoplasms found in the duodenum (1,2). If resectable, patients are generally treated with a pancreaticoduodenectomy (Whipple’s procedure) (3). We report a patient with a duodenal adenocarcinoma that was successfully resected by pancreas-sparing duodenectomy.
CASE REPORT
A 90-year-old female was transferred to our centre from a peripheral hospital. Her past medical history was significant for hypertension, mild cerebral vascular accidents, atrial fibrillation, and coronary artery disease. The patient had vomiting for more than 6 weeks. One week prior to transfer, she was intolerant of oral feeds, requiring parenteral feeds for nutrition. All vital signs were stable. Her abdomen was distended. She had epigastric tenderness, but no peritoneal signs. A nasogastric tube was inserted and 1.5 L of gastric fluid was immediately collected. Bloodwork on admission were as follows: haemoglobin 12.6g/dl; leukocyte count, 11500/mm3; creatinine 76 µmol/L. A Computed Tomography (CT) scan revealed a mass in the duodenum measuring 6 x 3 cm. No enlarged lymph nodes were detected. An EGD with biopsy was subsequently done and confirmed the diagnosis adenocarcinoma in the 2nd/3rd part of the duodenum. Upon arrival to LHSC, her abdomen was distended. She had epigastric tenderness, but no peritoneal signs.
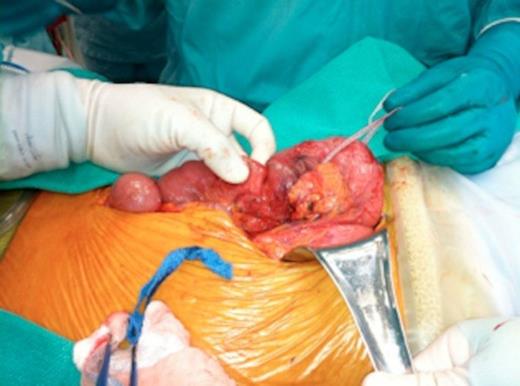
Intraoperative images of pancreas-sparing duodenectomy: Complete mobilization of duodenum and head of pancreas showing the tumour in the second/third portion of duodenum
After being assessed by the general surgery, medicine and anaesthesia teams, the patient was consented for a laparotomy. A midline incision was done and there were no signs of metastatic disease. The duodenal tumour was palpable in the 2nd/3rd portion of the duodenum. The right colon was mobilized and a Kocher manouver was performed (FiG. 1a). The tumour was located a few centimetres distal to the ampula of Vater. There was no macroscopic invasion into adjacent vessels or structures. No lymph nodes were palpable. The ligament of Treitz was incised and the proximal jejunum was passed beneath the superior mesenteric vessels to the right upper quadrant. The proximal jejunum was transected using a gastrointestinal stapler. Using sequential hemo-clips and silk ties, the duodenum was carefully dissected and separated from the pancreatic head. All feeding vessels were taken down from the uncinate process and ligated on the pancreatic side. The proximal margin of the duodenum was transected at the level of the second portion, approximately 2 cm above the tumour, next to the ampulla of Vater. The specimen was then removed and sent for pathology.
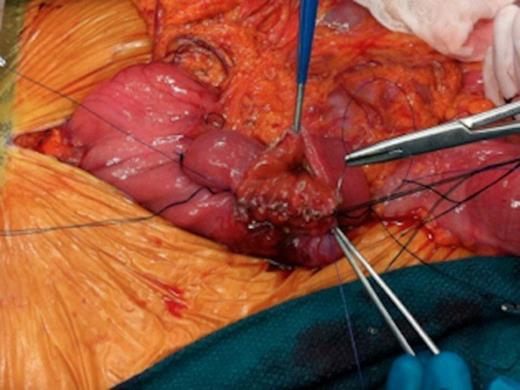
Intraoperative images of pancreas-sparing duodenectomy: Hand sewn, side to side, duodenojejunal anastomosis
A hand sewn side-to-side anastomosis between the second portion of the duodenum and the jejunum was performed (Fig. 1b and 1c). The anastomosis was sewn in two layers using a running and locking 3-0 PDS stitch for the inner layer, followed by a 3-0 silk for a second layer. Simple interrupted 3-0 silk sutures were used to tack down the jejunum at the ligament of Treitz. A suction drain was placed in the proximity of the anastomotic site and the abdomen closed. The operative time was three hours and the intraoperative blood loss was estimated to be 200ml.
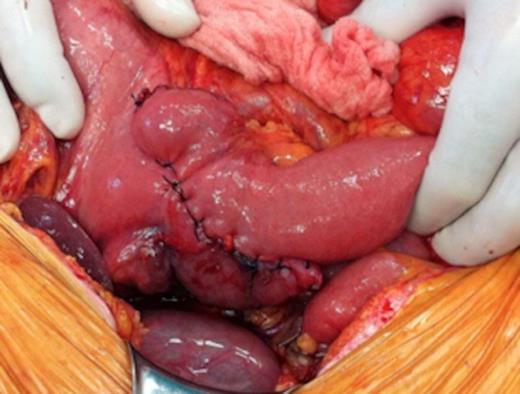
Intraoperative images of pancreas-sparing duodenectomy: completed anastomosis
Pathohistological analysis of the resected specimen revealed a low grade circumferential adenocarcinoma measuring 6.5cm x 3.5cm x 1.0 cm. The distance from the proximal duodenal cut line to the tumour was 2.2 cm. There was invasion through the muscularis propria, but the serosa was spared. Several mesenteric lymph nodes were identified and all were found negative for metastatic disease. No vascular invasion was identified.
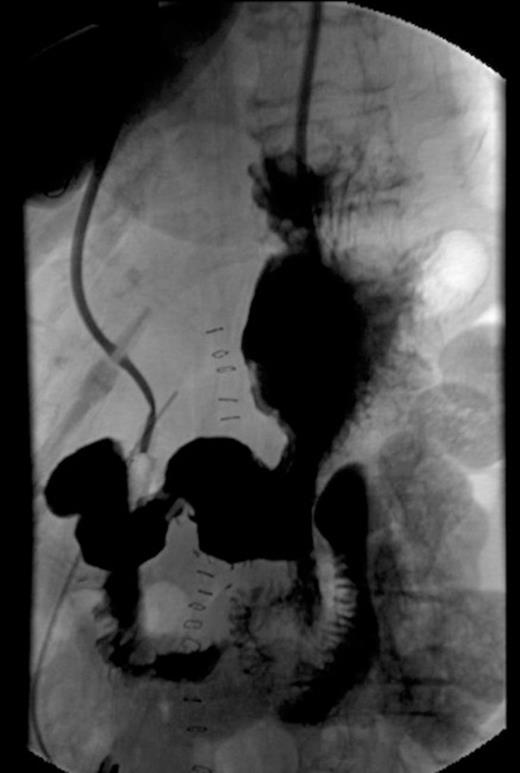
Post-operative UGI series showing no anastomotic leaks and patency of anastomosis.
The patient had an uneventful postoperative course. Initially, her nasogastric tube was kept in place and her parenteral nutrition was maintained. There were no clinical or biochemical signs of a pancreatic leak. A postoperative upper GI series was done on day 6 and the anastomosis was in-tact (Fig. 2). There were no leaks. After the contrast study, her suction drain was removed. At day 7, the patient was tolerating a transitional diet. By day 9, she was tolerating a full diet and discharged home. At her six month followup, the patient was doing well with no complaints. Oncology suggested no chemotherapy at the time.
DISCUSSION
Duodenal adenocarcinomas are rare and account for just 0.3% of all gastrointestinal malignancies (1,2). However, it is the most common neoplasm of the duodenum, accounting for 64% (4). Due to its non-specific symptoms, it is often not diagnosed until disease advanced. Surgical resection with removal of all invaded tissues is considered the best treatment, and in most instances a Whipple’s procedure is the treatment of choice for duodenal malignancies (3).
Small studies have shown that in select cases, pancreas-sparing partial duodectomy provides an acceptable morbidity and mortality rate for distal tumours located in the distal D3 or D4 when compared to pancreaticoduodenectomy (5). Lymph node invasion is a very important indicator for long term survival, more important than tumour size, or local invasion (6,7). Patients with lymph node metastases have 2-year survival rates of 30%, whereas patients those with no lymph node involvement have a 5-year survival rate of 72% (P<0.05) (8).
Pancreas sparing duodenectomy decreases operative time, avoids resection of distal bile duct and pancreas, and circumvents the subsequent need for biliary-enteric and pancreatic-anastomoses, and thus decreases the morbidity significantly (9).
Segmental resection of distal duodenal tumours have been demonstrated to be a safe alternative to pancreaticoduodenectomy (10).With our patient who was of advanced age and had significant medical comorbidities, a pancreaticoduodenectomy procedure would likely have been poorly tolerated. However, since there was no evidence of lymphatic, vascular, or extra luminal invasion, it was felt that this patient was potentially curable with complete resection. Pancreas-sparing duodenectomy was successfully performed and an R0 resection was confirmed by pathology. The patient has since been doing very well. Pancreas-sparing duodenectomy can be a safe alternative for D3-D4 duodenal tumours, particularly in select patients unlikely to tolerate a pancreaticoduodenectomy.



