-
PDF
- Split View
-
Views
-
Cite
Cite
SS Rajan, M. Saeed, M. Mestrah, Ductal carcinoma of the breast metastasizing to the rectum, Journal of Surgical Case Reports, Volume 2012, Issue 5, May 2012, Page 12, https://doi.org/10.1093/jscr/2012.5.12
Close - Share Icon Share
Abstract
Gastrointestinal metastasis of the breast cancer is rare and its management varies significantly from that of a primary bowel cancer. We report a case of invasive ductal cancer metastasizing to the rectum and masquerading as a rectal primary.
A 60 year old caucasian woman presented with fresh rectal bleeding nine years after treatment of her primary breast cancer. The investigations revealed features suggestive of primary rectal malignancy and was managed accordingly. However, the surgical histopathology revealed poorly differentiated metastatic adenocarcinoma and the immunohistochemical evaluation confirmed origin from a breast primary. She had an uneventful post-operative recovery and remains disease free thus far.
The continuing advancement in the management of breast cancer patients with resulting increase in the overall survival will lead to such unusual metastatic presentations. Hence, the awareness, identification and differentiation of such rare metastatic presentation are important in order to manage the patients appropriately in the future.
INTRODUCTION
Over the past few decades, the advancement in breast screening and management has increased the overall survival in women with breast cancer. But this in turn, exposes them to the risk of developing new primary malignancies or metastases. The metastatic involvement of gastrointestinal (GI) tract is rare and that of the rectum even rarer. Even though lobular carcinoma constitutes only 10% of breast cancers, it is the commonest breast cancer metastasizing to the colon and rectum (1). These metastatic lesions can lead to diagnostic challenge for clinicians as they can mimic primary colo-rectal cancer due to the lack of diagnostic signs. We report a rather unusual case of metastatic invasive ductal carcinoma masquerading as primary rectal cancer.
CASE REPORT
A 60 year old woman presented with a suspicious lump in her left breast in early 1996 and underwent open diagnostic surgical excision. She was found to have a moderately differentiated invasive ductal carcinoma measuring up to 20 mm in its greatest diameter extending less than 1 mm from the diagnostic excision margin. Subsequently, she underwent left mastectomy and axillary node clearance which failed to show any residual invasive cancer. However, there was residual high grade cribriform DCIS present adjacent to the diagnostic surgical excision cavity in the mastectomy specimen. There was no evidence of vascular invasion and none of the seven lymph nodes excised contained any metastatic carcinoma [G2T1N0Mx]. Since her cancer was oestrogen receptor (ER) positive, she was started on Tamoxifen post-operatively.
She presented 2 years later with localised pain in her right knee and bone scan revealed an isolated bony metastasis in the right proximal tibia. This was managed using palliative radiotherapy to control the symptoms and her adjuvant hormonal therapy was switched to Anastrazole (AstraZeneca plc, London, United Kingdom). She neither developed any loco-regional recurrence nor had any progression of her metastatic disease for the next 9 years. During this period, she underwent annual mammographic and clinical follow-up with her adjuvant hormonal treatment being stopped in 2005 after clinical consultation. In early 2007, she presented with ongoing history of fresh rectal bleeding, alternating bowel habit and mucus discharge per rectum. Her examination failed to demonstrate any anaemia, jaundice or generalised lymphadenopathy. Abdomen was soft, non-tender without any organomegaly and digital rectal examination revealed a palpable tumour at 6 o'clock position. Examination of her left mastectomy scar, right breast and axillae were unremarkable.
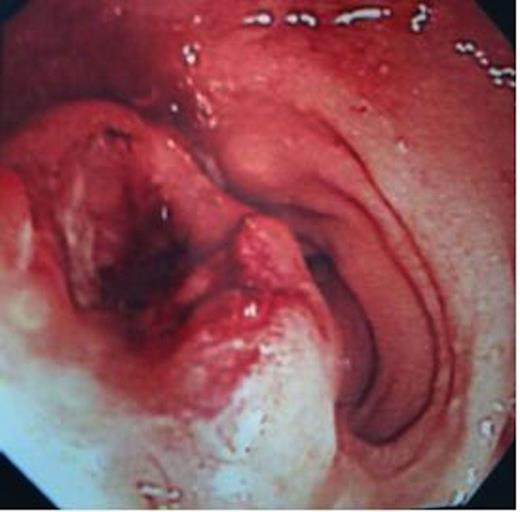
She underwent colonoscopy, which revealed a fungating tumour at 10 cm from the anal verge in the rectum and rest of the colon was normal (Figure 1). The histopathological examination of the biopsy revealed a poorly differentiated invasive adenocarcinoma (Figure 2).
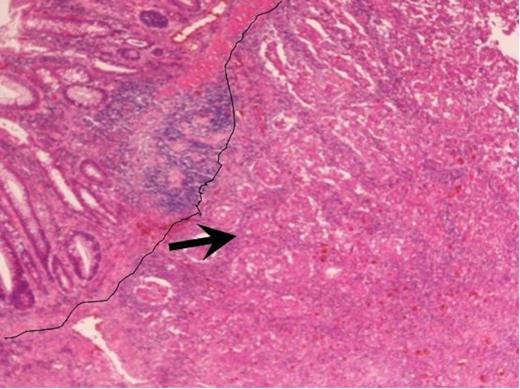
Haematoxylin and Eosin staining of the biopsy specimen. Tumour overlying the large bowel mucosa with the line demarcating the tumour edge with arrow pointing at the body of the tumour (magnification×4).
Considering her previous history of invasive breast cancer, pathologist performed immunohistochemistry of the colonic biopsies. The tumour cells were strongly positive for Cytokeratin (CK) 7 with focal positivity for ER but were negative for both Carcino Embryonic Antigen (CEA) and CK 20. The above immunohistochemistry profile with previous history of breast cancer favoured a metastatic breast origin over a primary colonic cancer even though the clinical and colonoscopic findings were not typical. The staging CT scan didn’t show any evidence of metastasis and the MRI scan revealed a locally advanced tumour with involvement of mesorectal facial margins with possible involvement of the peritoneum locally (Figure 3). The bone scan failed to reveal any evidence of active metastatic disease.
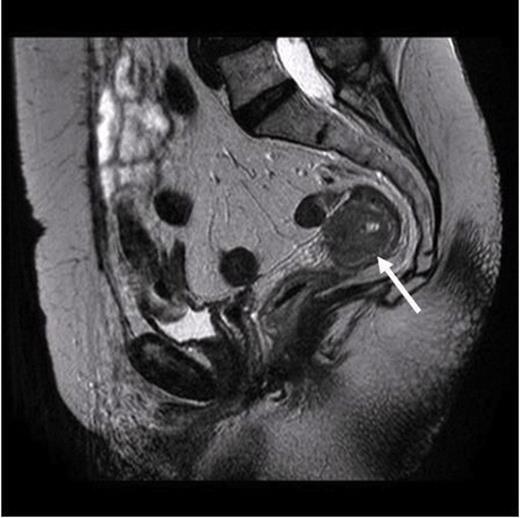
MRI scan. Arrow pointing to the localized tumour in the rectum with no evidence of widespread metastasis
After discussing in the multi-disciplinary team meeting (MDT), it was decided to treat the tumour with a short course of pre-operative radiotherapy. After radiotherapy, she underwent a low anterior resection with end colostomy formation. The histopathological examination of the resected tumour showed poorly differentiated metastatic adenocarcinoma with predominantly solid, but with focal glandular growth pattern and large areas of necrosis. The tumour involved full thickness of the bowel but the serosal surface and the resection margins were free of cancer. There was no evidence of metastatic cancer in any of the twenty two lymph nodes excised.
Immunohistochemistry showed tumour cells to be negative for CEA and CK 20, but was strongly positive for CK7 (Figure 4 & 5). The tumour also showed focal positivity for ER and was negative for both progesterone receptor (PR) and Human Epidermal Growth Factor Receptor 2 (Her2). After comparing the morphological and immunohistochemistry charachteristics of the primary breast cancer and rectal tumour, the pathologist concluded that the features were consistent with a primary breast cancer metastasising to the rectum. She underwent an uneventful post-operative recovery and remains disease free during her follow-up until now.
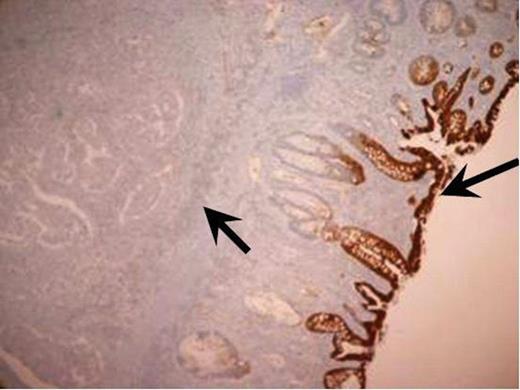
Immunohistochemistry staining of the resected tumour. CK 20 immunohistochemistry positive normal large bowel mucosa with the adenocarcinoma being negative (magnification × 4).
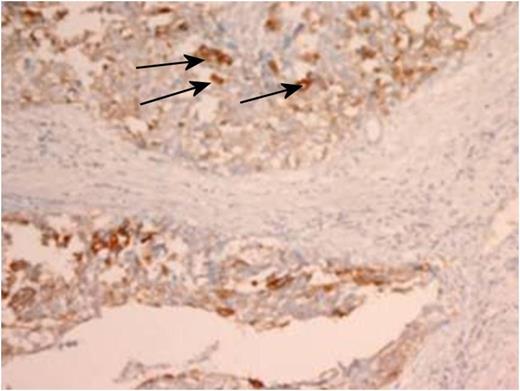
Immunohistochemistry staining of the resected tumour. Arrows identifying positive CK7 staining within tumour (magnification × 4).
DISCUSSION
In the largest clinical series till date, only <1% (17 out of 2604 patients followed over 18 years) of the patients with breast cancer were found to have Gastro-intestinal [GI] metastasis (2). Information gathered from autopsy series shows small intestine being the commonest site of metastasis (28%) followed by Oesophagus (25%), Stomach (25%), Colon (19%) and Rectum (4%). The commonest route of metastasis to the GI tract is through the haematogenous route followed by lymphatic and peritoneal spread. Even though there are a few case reports of lobular carcinoma metastasizing to the rectum, there are only 3 case reports in the literature of invasive ductal carcinoma metastasizing to the rectum till date (3-5).
As a clinician the main challenge lies in the differentiation of such metastatic lesions from primary colo-rectal cancer as the management varies significantly. The clinical presentation of metastatic lesion can be non-specific and mimic a primary cancer as in the present case. The radiological investigations like, CT scan and double contrast barium enema are helpful in localizing the lesion but cannot differentiate between them.
On endoscopy, the colo-rectal metastasis appear as diffuse thickening of the colonic wall mimicking linitis plastica or like in crohn’s disease with ulcerated or nodular areas rather than as a solitary, discrete mass seen in primary colonic lesion (1). But the differentiation was made more challenging in our case, as colonoscopy revealed a solitary fungating mass arising from the rectal mucosa mimicking a primary rectal cancer. Histopathological examination by itself may not be conclusive as the tumour invasion is primarily in the sub-serosa, so unless deep endoscopic biopsies are available the diagnosis will be made only after resection of the tumour. But immunohistochemical markers such as ER, PR, gross cystic disease fluid protein (GCDFP-15), and differential expression of CK7 and CK20 can facilitate an accurate diagnosis as seen in the present report.
Systemic treatment with chemotherapy and/or hormonal therapy is usually employed in patients with confirmed diagnosis of gastrointestinal metastasis (1). The role of surgery is limited to palliation or in patients presenting acutely with obstruction or perforation of the hollow viscus. The lack of concordance between the clinical, radiological and colonoscopic findings with that of the histological findings resulted in our patient being managed with pre-operative radiotherapy followed with low anterior resection. The prognosis of GI metastasis from primary breast cancer is poor with few patients surviving beyond two years, although survival up to nine years has been reported. Our patient remains well and disease free four years after treatment for her rectal metastasis.
Given the increased survival of breast cancer patients due to the early diagnosis through breast screening and better management with current therapeutic regimens, more unusual presentations of metastatic disease, including involvement of the gastrointestinal tract should be anticipated. So the recognition of this rare entity (rectal metastasis from primary breast cancer) is important, as presentation resembles that of primary rectal carcinoma and differentiation is vital as different therapeutic modalities may be appropriate.



