-
PDF
- Split View
-
Views
-
Cite
Cite
Sermsak Sukpanichyingyong, Krits Salang, Thananit Sangkomkamhang, Clinical outcomes of extended intralesional curettage with cementation without implant augmentation in non-fracture giant cell tumor of bone around the knee, Journal of Surgical Case Reports, Volume 2022, Issue 4, April 2022, rjac197, https://doi.org/10.1093/jscr/rjac197
Close - Share Icon Share
Abstract
There remains a lack of consensus regarding the necessity of implant augmentation or fixation after intralesional curettage in giant cell tumor of bone (GCTB) around the knee. This study assessed whether cementation alone is effective and safe in GCTB with a non-fracture around the knee. We retrospectively examined clinical data from 14 GCTB patients treated from 2012 to 2022. Outcome parameters were Musculoskeletal Tumor Society (MSTS) score, postoperative fracture, metastases, recurrence and complications. Of the 14 GCTB cases examined, 10 were at the distal femur and four were at the proximal tibia. Mean patient age was 32 years, and follow-up time was 61 months. Mean tumor size was 61 × 79 × 50 mm, and MSTS score was 89.2%. There were no cases of postoperative fracture. Defect reconstruction with cementation alone may be strong enough to provide immediate stability and prevent postoperative fracture in GCTB around the knee.
INTRODUCTION
Giant cell tumor of bone (GCTB) is a benign aggressive bone tumor that represents 3–8% of all primary bone tumors worldwide and 20% in Asian countries [1]. In such cases, operative management is usually necessary. Intralesional curettage is the most common surgical approach in GCTB, as it allows for greater preservation the bone and adjacent joint and provides better functional outcomes [2]. Following intralesional curettage, high-speed burring is commonly performed to extend the surgical margins, as well as filling with polymethyl methacrylate (PMMA), bone grafting or bone substitution, and the application of chemical or thermal adjuvants (hydrogen peroxide, phenol, PMMA, liquid nitrogen, alcohol and argon beam coagulation). A previous study found that burring, the use of chemical/thermal adjuvants and cementation decreased the rate of recurrence by 0–26% [3].
There remains a lack of consensus concerning what type of cavity filling (PMMA vs bone grafting; [4, 5]) or additional adjuvant (chemicals or thermal; [6]) should be used, as well as regarding the need for implant augmentation or fixation when intralesional curettage is performed [7]. There are currently no indications for the use of implant augmentation or internal fixation in GCTB. This is particularly true in cases in which the tumor is located around the knee joint, which is a weight-bearing joint involved in extensive activity. Several reports have recommended implant augmentation or internal fixation, such as pins, screws, nails or plates, to reduce the postoperative fracture, prevent micromotion between the bone and cement, and promote early improvement with regard to range-of-motion [8–10]. The purpose of our study was to report clinical outcomes of extended intralesional curettage with cementation without implant augmentation or internal fixation in non-fracture around the knee.
CASE SERIES
The institutional review board approved this retrospective study and waived the requirement for patient informed consent. Between January 2012 and June 2022, a total of 14 cases of GCTB around the knee (distal femur and proximal tibia) were diagnosed. Clinical data were recorded including age, sex, tumor size and site, ratio of the greatest tumor and bone diameters, Campanacci radiographic classification [11], subchondral bone involvement, metastases, surgical procedures, Musculoskeletal Tumor Society (MSTS) functional score [12], postoperative fracture, recurrence, duration of follow-up in months and complications.
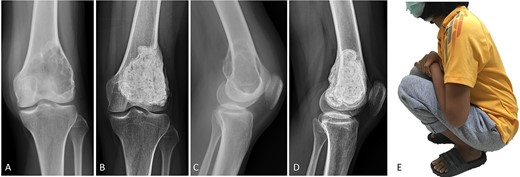
(A, C) Case no. 5: female, 23 years of age with GCTB at the distal femur without subchondral bone involvement, Campanacci grade III. (B, D) Radiograph at 81 months after extended curettage with hydrogen peroxide, phenol and cementation. (E) MSTS score 93%.
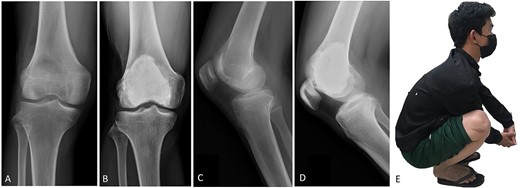
(A, C) Case no. 6: male, 15 years of age with GCTB at the distal femur without subchondral bone involvement, Campanacci grade II. (B, D) Radiograph at 67 months after extended curettage with hydrogen peroxide, phenol, and cementation. (E) MSTS score 100%.
Surgical procedures were performed by two orthopedic oncology surgeons using the same surgical technique. In all cases, GCTB was confirmed by clinical data, plain radiography and magnetic resonance imaging (MRI), as well as histopathologic examination before and after surgery. Whether a medial or lateral approach was employed depended on tumor location. A large cortical window equal in size to the tumor area was created to provide visualization of the entire tumor cavity, and intralesional curettage of the tumor was performed. If the tumor extended into the soft tissue, the entire pseudo-capsule was dissected circumferentially and excised. A high-speed burr was used to extend the cavity by at least 2 mm to remove the residual tumor in all cases except those in which the lesion was in subchondral bone and cartilage. Hydrogen peroxide is the preferred local adjuvant agent at our institution, but phenol was applied along the cavity wall using gauze in three cases, and argon beam coagulator was applied in six. After the application of local adjuvant, the irrigation cavity was rinsed with saline solution. If the tumor involved subchondral bone or cartilage, hydroxyapatite bone substitution was applied 5–10-mm above subchondral bone to prevent complications from allograft and donor-site morbidity of the autogenous bone graft. We defined subchondral bone involvement as tumor location <5 mm from joint cartilage. Every part of the remaining cavity was filled with PMMA without implant augmentation or internal fixation (Figs 1–4). The large cortical window was left open with cement in most of the cases in which the cortex was destroyed by the tumor.
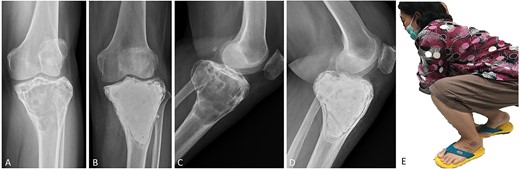
(A, C) Case no. 7: female, 54 years of age with GCTB at the proximal tibia with subchondral bone involvement, Campanacci grade III. (B, D) Radiograph at 58 months after extended curettage with hydrogen peroxide, phenol, and cementation. (E) MSTS score 86%.
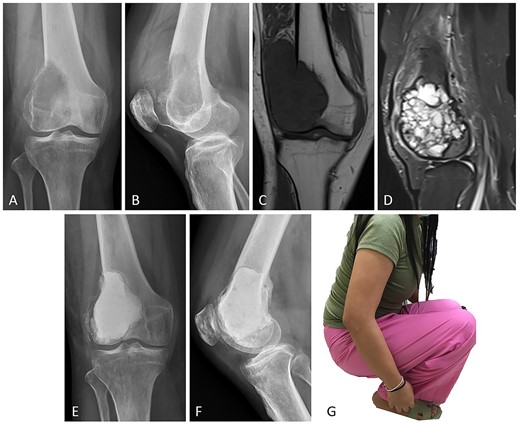
Case no. 13: (A–B) male, 32 years of age with GCTB at the distal femur with subchondral bone involvement, Campanacci grade III. (C–D) Preoperative T1-weighted coronal view MRI showing low-intensity with soft tissue extension and T2-weighted sagittal view MRI showing a heterogeneous high-intensity change with surrounding soft-tissue edema. (E–F) Radiograph at 26 months after extended curettage with hydrogen peroxide, argon beam coagulation, and cementation with hydroxyapatite bone substitution (packed above the subchondral bone). (G) MSTS score 86%.
All patients were instructed to refrain from putting weight on the joint for 2 weeks, followed by tolerated, partial weight-bearing for 2 weeks in those with intact subchondral bone and 3–4 weeks in those with subchondral bone involvement. A range-of-motion and muscle-strengthening exercise regimen was initiated postoperatively. The follow-up protocol consisted of clinical examination and radiography to detect local recurrences or complications at 1, 2 and 3 months postoperatively and then half-yearly for 2 years and yearly thereafter.
We examined the data of 14 GCTB patients without fracture (8 females and 6 males) with a mean age of 32 years (range, 15–54 years). The lesion was located at the distal femur in 10 cases and proximal tibia four. There were 11 cases with grade III lesions and 3 with grade II lesions according to Campanacci’s radiographic classification system (Table 1). The mean ratio of the greatest tumor/bone anteroposterior diameter was 72% (range, 50–92%) and lateral diameter was 79% (range, 64–95%). There was tumor invasion to the subchondral bone in eight cases. The mean follow-up period was 61 months (range, 24–113 months). There was local recurrence in one case at the distal femur after 7 months, which was treated by extended curettage with cementation. Complications associated with surgery occurred in one patient, who presented with a superficial wound infection that resolved with debridement and antibiotics. No fracturing occurred in any of the patients, and the mean MSTS score at the last follow-up was 89.2% (range, 76–100%).
| Patient no. . | Age, y . | Sex . | Site of tumor . | Size of tumor, mm . | The ratio of the greatest diameter (AP, Lat), % . | Campanacci grade . | Subchondral bone involvement . | Local adjuvant . | Follow-up, mo . | MSTS score, % . | Complications . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 50 | M | PT | 64 × 81 × 70 | 65, 70 | III | No | H2O2 | 113 | 93 | No |
| 2 | 43 | M | PT | 63 × 82 × 54 | 77, 71 | III | No | H2O2 | 93 | 100 | No |
| 3 | 22 | M | DF | 51 × 106 × 57 | 73, 71 | III | Yes | H2O2 | 93 | 86 | Wound infection |
| 4 | 30 | F | DF | 56 × 73 × 52 | 75, 67 | III | No | H2O2 | 91 | 93 | No |
| 5 | 23 | F | DF | 54 × 57 × 41 | 76, 64 | III | No | H2O2, Phenol | 81 | 93 | No |
| 6 | 15 | M | DF | 65 × 72 × 43 | 58, 73 | II | No | H2O2, Phenol | 67 | 100 | No |
| 7 | 54 | F | PT | 76 × 79 × 76 | 91, 95 | III | Yes | H2O2, Phenol | 58 | 86 | No |
| 8 | 37 | F | DF | 68 × 80 × 38 | 92, 90 | III | Yes | H2O2 | 52 | 90 | No |
| 9 | 32 | F | DF | 60 × 62 × 40 | 70, 75 | II | No | H2O2, Argon beam | 46 | 93 | No |
| 10 | 29 | F | PT | 57 × 87 × 49 | 72, 73 | II | Yes | H2O2, Argon beam | 43 | 86 | No |
| 11 | 20 | M | DF | 78 × 95 × 50 | 68, 90 | III | Yes | H2O2, Argon beam | 38 | 76 | No |
| 12 | 43 | F | DF | 63 × 97 × 43 | 69, 83 | III | Yes | H2O2, Argon beam | 30 | 83 | Local recurrence |
| 13 | 32 | M | DF | 45 × 74 × 51 | 50, 90 | III | Yes | H2O2, Argon beam | 26 | 86 | No |
| 14 | 14 | F | DF | 54 × 67 × 40 | 73, 89 | III | Yes | H2O2, Argon beam | 24 | 90 | No |
| Patient no. . | Age, y . | Sex . | Site of tumor . | Size of tumor, mm . | The ratio of the greatest diameter (AP, Lat), % . | Campanacci grade . | Subchondral bone involvement . | Local adjuvant . | Follow-up, mo . | MSTS score, % . | Complications . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 50 | M | PT | 64 × 81 × 70 | 65, 70 | III | No | H2O2 | 113 | 93 | No |
| 2 | 43 | M | PT | 63 × 82 × 54 | 77, 71 | III | No | H2O2 | 93 | 100 | No |
| 3 | 22 | M | DF | 51 × 106 × 57 | 73, 71 | III | Yes | H2O2 | 93 | 86 | Wound infection |
| 4 | 30 | F | DF | 56 × 73 × 52 | 75, 67 | III | No | H2O2 | 91 | 93 | No |
| 5 | 23 | F | DF | 54 × 57 × 41 | 76, 64 | III | No | H2O2, Phenol | 81 | 93 | No |
| 6 | 15 | M | DF | 65 × 72 × 43 | 58, 73 | II | No | H2O2, Phenol | 67 | 100 | No |
| 7 | 54 | F | PT | 76 × 79 × 76 | 91, 95 | III | Yes | H2O2, Phenol | 58 | 86 | No |
| 8 | 37 | F | DF | 68 × 80 × 38 | 92, 90 | III | Yes | H2O2 | 52 | 90 | No |
| 9 | 32 | F | DF | 60 × 62 × 40 | 70, 75 | II | No | H2O2, Argon beam | 46 | 93 | No |
| 10 | 29 | F | PT | 57 × 87 × 49 | 72, 73 | II | Yes | H2O2, Argon beam | 43 | 86 | No |
| 11 | 20 | M | DF | 78 × 95 × 50 | 68, 90 | III | Yes | H2O2, Argon beam | 38 | 76 | No |
| 12 | 43 | F | DF | 63 × 97 × 43 | 69, 83 | III | Yes | H2O2, Argon beam | 30 | 83 | Local recurrence |
| 13 | 32 | M | DF | 45 × 74 × 51 | 50, 90 | III | Yes | H2O2, Argon beam | 26 | 86 | No |
| 14 | 14 | F | DF | 54 × 67 × 40 | 73, 89 | III | Yes | H2O2, Argon beam | 24 | 90 | No |
Note: AP: anteroposterior, Lat: lateral, DF: distal femur, PT: proximal tibia, H2O2: hydrogen peroxide, subchondral bone involvement: distance to the tumor <5 mm from joint cartilage.
| Patient no. . | Age, y . | Sex . | Site of tumor . | Size of tumor, mm . | The ratio of the greatest diameter (AP, Lat), % . | Campanacci grade . | Subchondral bone involvement . | Local adjuvant . | Follow-up, mo . | MSTS score, % . | Complications . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 50 | M | PT | 64 × 81 × 70 | 65, 70 | III | No | H2O2 | 113 | 93 | No |
| 2 | 43 | M | PT | 63 × 82 × 54 | 77, 71 | III | No | H2O2 | 93 | 100 | No |
| 3 | 22 | M | DF | 51 × 106 × 57 | 73, 71 | III | Yes | H2O2 | 93 | 86 | Wound infection |
| 4 | 30 | F | DF | 56 × 73 × 52 | 75, 67 | III | No | H2O2 | 91 | 93 | No |
| 5 | 23 | F | DF | 54 × 57 × 41 | 76, 64 | III | No | H2O2, Phenol | 81 | 93 | No |
| 6 | 15 | M | DF | 65 × 72 × 43 | 58, 73 | II | No | H2O2, Phenol | 67 | 100 | No |
| 7 | 54 | F | PT | 76 × 79 × 76 | 91, 95 | III | Yes | H2O2, Phenol | 58 | 86 | No |
| 8 | 37 | F | DF | 68 × 80 × 38 | 92, 90 | III | Yes | H2O2 | 52 | 90 | No |
| 9 | 32 | F | DF | 60 × 62 × 40 | 70, 75 | II | No | H2O2, Argon beam | 46 | 93 | No |
| 10 | 29 | F | PT | 57 × 87 × 49 | 72, 73 | II | Yes | H2O2, Argon beam | 43 | 86 | No |
| 11 | 20 | M | DF | 78 × 95 × 50 | 68, 90 | III | Yes | H2O2, Argon beam | 38 | 76 | No |
| 12 | 43 | F | DF | 63 × 97 × 43 | 69, 83 | III | Yes | H2O2, Argon beam | 30 | 83 | Local recurrence |
| 13 | 32 | M | DF | 45 × 74 × 51 | 50, 90 | III | Yes | H2O2, Argon beam | 26 | 86 | No |
| 14 | 14 | F | DF | 54 × 67 × 40 | 73, 89 | III | Yes | H2O2, Argon beam | 24 | 90 | No |
| Patient no. . | Age, y . | Sex . | Site of tumor . | Size of tumor, mm . | The ratio of the greatest diameter (AP, Lat), % . | Campanacci grade . | Subchondral bone involvement . | Local adjuvant . | Follow-up, mo . | MSTS score, % . | Complications . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 50 | M | PT | 64 × 81 × 70 | 65, 70 | III | No | H2O2 | 113 | 93 | No |
| 2 | 43 | M | PT | 63 × 82 × 54 | 77, 71 | III | No | H2O2 | 93 | 100 | No |
| 3 | 22 | M | DF | 51 × 106 × 57 | 73, 71 | III | Yes | H2O2 | 93 | 86 | Wound infection |
| 4 | 30 | F | DF | 56 × 73 × 52 | 75, 67 | III | No | H2O2 | 91 | 93 | No |
| 5 | 23 | F | DF | 54 × 57 × 41 | 76, 64 | III | No | H2O2, Phenol | 81 | 93 | No |
| 6 | 15 | M | DF | 65 × 72 × 43 | 58, 73 | II | No | H2O2, Phenol | 67 | 100 | No |
| 7 | 54 | F | PT | 76 × 79 × 76 | 91, 95 | III | Yes | H2O2, Phenol | 58 | 86 | No |
| 8 | 37 | F | DF | 68 × 80 × 38 | 92, 90 | III | Yes | H2O2 | 52 | 90 | No |
| 9 | 32 | F | DF | 60 × 62 × 40 | 70, 75 | II | No | H2O2, Argon beam | 46 | 93 | No |
| 10 | 29 | F | PT | 57 × 87 × 49 | 72, 73 | II | Yes | H2O2, Argon beam | 43 | 86 | No |
| 11 | 20 | M | DF | 78 × 95 × 50 | 68, 90 | III | Yes | H2O2, Argon beam | 38 | 76 | No |
| 12 | 43 | F | DF | 63 × 97 × 43 | 69, 83 | III | Yes | H2O2, Argon beam | 30 | 83 | Local recurrence |
| 13 | 32 | M | DF | 45 × 74 × 51 | 50, 90 | III | Yes | H2O2, Argon beam | 26 | 86 | No |
| 14 | 14 | F | DF | 54 × 67 × 40 | 73, 89 | III | Yes | H2O2, Argon beam | 24 | 90 | No |
Note: AP: anteroposterior, Lat: lateral, DF: distal femur, PT: proximal tibia, H2O2: hydrogen peroxide, subchondral bone involvement: distance to the tumor <5 mm from joint cartilage.
Factors that were associated with lower MSTS scores were subchondral bone involvement and a ratio of the greatest diameter in the lateral view (Table 2). Subchondral bone involvement was predictive of MSTS score according to multivariate analysis after adjustment for age, sex, tumor grade and tumor volume (Table 3). Stata version 10.1 (Stata Corp., College Station, TX, USA) was used for the analysis of our data. Univariate and multivariate (two variables) regression analysis was performed to determine independent factors of influence on functional outcomes (MSTS score). From plain radiograph, we defined subchondral bone involvement when the distance from joint cartilage to the tumor was <5 mm and the ratio of the greatest diameter was measured by dividing the maximal transverse osteolytic lesion by the maximal transverse bone length in anteroposterior and lateral view. P-values <0.05 were considered statistically significant.
| . | . | Mean difference (95%CI) . | P-value . |
|---|---|---|---|
| Age | 31.71 ± 12.48 yrs. | 0.01 (−0.34 to 0.35) | 0.969 |
| Sex | 0.983 | ||
| Male | 42.86% | 0 | |
| Female | 57.14% | 0.08 (−8.38 to 8.54) | |
| Location of tumor | 0.508 | ||
| Distal femur | 71.43% | 0 | |
| Proximal tibia | 29.57% | 2.85 (−6.24 to 11.94) | |
| Grade of tumor | 0.302 | ||
| Grade II | 21.43% | 0 | |
| Grade III | 78.57% | −4.82 (−14.56 to 4.93) | |
| Tumor volume | 249 474 ± 97 263 mm3 | −0.00002 (−0.00006 to 0.00002) | 0.290 |
| The ratio of the greatest diameter in AP view | 72.07 ± 10.99% | 0.08 (−0.31 to 0.47) | 0.674 |
| The ratio of the greatest diameter in lateral view | 78.64 ± 10.37% | −0.4 (−0.74 to −0.07) | 0.022* |
| Local adjuvant | 0.093 | ||
| H2O2 | 35.71% | 0 | |
| H2O2, phenol | 21.43% | 0.6 (−9.12 to 10.32) | |
| H2O2, Argon beam | 42.86% | −7.73 (−15.8 to 0.33) | |
| Subchondral bone involvement | 0.001* | ||
| No | 57.14% | 0 | |
| Yes | 42.86% | −10.71 (−15.83 to −5.59) |
| . | . | Mean difference (95%CI) . | P-value . |
|---|---|---|---|
| Age | 31.71 ± 12.48 yrs. | 0.01 (−0.34 to 0.35) | 0.969 |
| Sex | 0.983 | ||
| Male | 42.86% | 0 | |
| Female | 57.14% | 0.08 (−8.38 to 8.54) | |
| Location of tumor | 0.508 | ||
| Distal femur | 71.43% | 0 | |
| Proximal tibia | 29.57% | 2.85 (−6.24 to 11.94) | |
| Grade of tumor | 0.302 | ||
| Grade II | 21.43% | 0 | |
| Grade III | 78.57% | −4.82 (−14.56 to 4.93) | |
| Tumor volume | 249 474 ± 97 263 mm3 | −0.00002 (−0.00006 to 0.00002) | 0.290 |
| The ratio of the greatest diameter in AP view | 72.07 ± 10.99% | 0.08 (−0.31 to 0.47) | 0.674 |
| The ratio of the greatest diameter in lateral view | 78.64 ± 10.37% | −0.4 (−0.74 to −0.07) | 0.022* |
| Local adjuvant | 0.093 | ||
| H2O2 | 35.71% | 0 | |
| H2O2, phenol | 21.43% | 0.6 (−9.12 to 10.32) | |
| H2O2, Argon beam | 42.86% | −7.73 (−15.8 to 0.33) | |
| Subchondral bone involvement | 0.001* | ||
| No | 57.14% | 0 | |
| Yes | 42.86% | −10.71 (−15.83 to −5.59) |
*Statistically significant.
| . | . | Mean difference (95%CI) . | P-value . |
|---|---|---|---|
| Age | 31.71 ± 12.48 yrs. | 0.01 (−0.34 to 0.35) | 0.969 |
| Sex | 0.983 | ||
| Male | 42.86% | 0 | |
| Female | 57.14% | 0.08 (−8.38 to 8.54) | |
| Location of tumor | 0.508 | ||
| Distal femur | 71.43% | 0 | |
| Proximal tibia | 29.57% | 2.85 (−6.24 to 11.94) | |
| Grade of tumor | 0.302 | ||
| Grade II | 21.43% | 0 | |
| Grade III | 78.57% | −4.82 (−14.56 to 4.93) | |
| Tumor volume | 249 474 ± 97 263 mm3 | −0.00002 (−0.00006 to 0.00002) | 0.290 |
| The ratio of the greatest diameter in AP view | 72.07 ± 10.99% | 0.08 (−0.31 to 0.47) | 0.674 |
| The ratio of the greatest diameter in lateral view | 78.64 ± 10.37% | −0.4 (−0.74 to −0.07) | 0.022* |
| Local adjuvant | 0.093 | ||
| H2O2 | 35.71% | 0 | |
| H2O2, phenol | 21.43% | 0.6 (−9.12 to 10.32) | |
| H2O2, Argon beam | 42.86% | −7.73 (−15.8 to 0.33) | |
| Subchondral bone involvement | 0.001* | ||
| No | 57.14% | 0 | |
| Yes | 42.86% | −10.71 (−15.83 to −5.59) |
| . | . | Mean difference (95%CI) . | P-value . |
|---|---|---|---|
| Age | 31.71 ± 12.48 yrs. | 0.01 (−0.34 to 0.35) | 0.969 |
| Sex | 0.983 | ||
| Male | 42.86% | 0 | |
| Female | 57.14% | 0.08 (−8.38 to 8.54) | |
| Location of tumor | 0.508 | ||
| Distal femur | 71.43% | 0 | |
| Proximal tibia | 29.57% | 2.85 (−6.24 to 11.94) | |
| Grade of tumor | 0.302 | ||
| Grade II | 21.43% | 0 | |
| Grade III | 78.57% | −4.82 (−14.56 to 4.93) | |
| Tumor volume | 249 474 ± 97 263 mm3 | −0.00002 (−0.00006 to 0.00002) | 0.290 |
| The ratio of the greatest diameter in AP view | 72.07 ± 10.99% | 0.08 (−0.31 to 0.47) | 0.674 |
| The ratio of the greatest diameter in lateral view | 78.64 ± 10.37% | −0.4 (−0.74 to −0.07) | 0.022* |
| Local adjuvant | 0.093 | ||
| H2O2 | 35.71% | 0 | |
| H2O2, phenol | 21.43% | 0.6 (−9.12 to 10.32) | |
| H2O2, Argon beam | 42.86% | −7.73 (−15.8 to 0.33) | |
| Subchondral bone involvement | 0.001* | ||
| No | 57.14% | 0 | |
| Yes | 42.86% | −10.71 (−15.83 to −5.59) |
*Statistically significant.
DISCUSSION
Surgery is the main treatment modality for GCTB. Wide resection with reconstruction provides better local control but causes loss of bone, cartilage and ligament, leading to functional impairment and late complications of reconstruction [13]. Extended intralesional curettage with or without an adjuvant is the most common treatment according to several previous reports [2, 3, 14]. The use of one or two physical, chemical or thermal adjuvants is recommended to prevent local recurrence after curettage [3]. After curettage, the cavity should be filled. There are several options for this including PMMA, bone grafting with autograft or allograft and bone substitution [15]. There are many unanswered questions regarding adjuvant use and defect reconstruction. The advantages of cementation as a filler for large defects are that it provides immediate stability and has exothermic properties resulting from the polymerization of PMMA [16]. At our institution, extended curettage with application of additional adjuvants and cementation is the primary treatment for GCTB (even Campanacci grade III) except in cases of enlarged tumor or multiple recurrences. In this study, we found one case of local recurrence (7%) at the distal femur, which was Campanacci grade III and was treated with extended curettage and hydrogen peroxide, argon beam coagulation and cementation as adjuvants.
| . | Adjust for age, sex, grade, tumor volume, the ratio of the greatest diameter in AP and lateral view, and local adjuvants . | |
|---|---|---|
| . | Mean difference (95%CI) . | P-value . |
| Subchondral bone involvement | 0.001* | |
| No | 0 | |
| Yes | −10.71 (−15.83 to −5.59) | |
| . | Adjust for age, sex, grade, tumor volume, the ratio of the greatest diameter in AP and lateral view, and local adjuvants . | |
|---|---|---|
| . | Mean difference (95%CI) . | P-value . |
| Subchondral bone involvement | 0.001* | |
| No | 0 | |
| Yes | −10.71 (−15.83 to −5.59) | |
*Statistically significant.
| . | Adjust for age, sex, grade, tumor volume, the ratio of the greatest diameter in AP and lateral view, and local adjuvants . | |
|---|---|---|
| . | Mean difference (95%CI) . | P-value . |
| Subchondral bone involvement | 0.001* | |
| No | 0 | |
| Yes | −10.71 (−15.83 to −5.59) | |
| . | Adjust for age, sex, grade, tumor volume, the ratio of the greatest diameter in AP and lateral view, and local adjuvants . | |
|---|---|---|
| . | Mean difference (95%CI) . | P-value . |
| Subchondral bone involvement | 0.001* | |
| No | 0 | |
| Yes | −10.71 (−15.83 to −5.59) | |
*Statistically significant.
There also remains a lack of consensus concerning the need for implant augmentation or internal fixation when intralesional curettage and cementation are performed. Several previous reports have recommended implant augmentation or internal fixation after curettage and cementation as these provide greater mechanical strength than cementation alone [8–10]. In theory, the risk of fracture can be reduced by augmenting the cement with internal fixation devices [17]. Fraquet et al. indicated that osteosynthesis could prevent the bead effect, in which bone remnants are displaced around the cement block, resulting treatment failure [9]. Wu et al. prefer a locking plate, which prevents micromotion between the bone and cement, thus improving the stability of the affected limb [18]. Ranu et al. suggested internal fixation around the knee joint if the subchondral bone thickness is <5 mm and articular surface involvement is >50% to prevent articular collapse [7]. In our study, there were no cases of postoperative fracture, bead effect, or failed defect reconstruction after extended curettage and cementation without osteosynthesis in patients in whom the tumor involved >50% of the bone (mean greatest anteroposterior and lateral tumor/bone ratio: 72 and 79%, respectively). We focused on lesions around the knee, which is a weight-bearing area and the locus of extensive activity. The advantage of PMMA as a filler for large defects is its mechanical properties, which are similar to human bone and stronger against compression [17]. Moreover, when PMMA is used for defect reconstruction, it results in subchondral stiffness at ~98% that of an intact contralateral limb, which prevents cartilage rarefaction and fracture of the subchondral bone [8, 18]. Based on our results, only cementation and adjuvant treatment with hydrogen peroxide, phenol or argon beam coagulation were strong enough to provide immediate stability and prevent postoperative fracture. Gupta et al. found that even in cases of pathological fracture, extended curettage and cementation without fixation resulted in satisfactory outcomes in GCTB patients [19]. The risk of postoperative fracture has been shown to be higher when cryosurgery is used as an adjuvant [20] and may result in the need for implant augmentation or fixation, which may involve more extensive surgery, more soft tissue contamination, greater cost or soft tissue irritation from plate fixation (especially in the medial proximal tibia, which may require implant removal). Moreover, due to the high tumor recurrence rate of GCTB, we should be concerned about metal-induced MRI artifacts, which may cause difficulties in interpretation and surgery planning, especially in cases of recurrence in soft tissue [21].
There is frequently subchondral involvement in GCTB, which is associated with poor functional outcomes. Chen et al. found that such outcomes were associated with larger affected area of the subchondral bone [22]. In our series, the mean MSTS score was 89.2% (range, 76–100%) with a mean follow-up period of 61 months. Univariate and multivariate analysis confirmed previous findings that patients with lower MSTS scores are more likely to have subchondral bone involvement. We found that neither age, sex, tumor size, tumor volume, Campanacci stage, nor adjuvant type had a significant effect on functional outcomes. Some previous reports have recommended the placement of an autogenous or allograft between the cartilage and PMMA to reduce the pressure on the cartilage and subchondral bone [4, 18]. However, there were no statistically significant differences in functional outcomes between patients who underwent bone grafting and those in whom cementation at the subchondral bone was performed after curettage [23]. At our institution, to prevent allograft-related complications and donor-site morbidity of the autogenous bone graft, we prefer hydroxyapatite bone substitution, which is packed adjacent to the subarticular surface. Many studies have shown that cementation may increase the stiffness of the subchondral bone, leading to secondary osteoarthritis in the adjacent joint and increasing the time needed for healing in local tissue [24]. However, this study did not examine this due to the short follow-up period.
There were several limitations to this study. The first was its retrospective nature, intrinsic to which are certain problems regarding data collection. Second, the rarity of GCTB meant that we were only able to examine a small number of cases, which might have affected the power of the statistical analysis of functional outcomes. Finally, due to the short follow-up period, we were unable to assess secondary osteoarthritis change. Large scale/multi-center randomized controlled trials are required to further explore cementation with and without implant augmentation or internal fixation in patients with GCTB around the knee.
CONCLUSIONS
Defect reconstruction with cementation without implant augmentation may be strong enough to provide immediate stability and prevent postoperative fracture after extended curettage of GCTB. Subchondral bone involvement is associated with lower functional outcomes.
CONFLICT OF INTEREST STATEMENT
The authors report no conflict of interest, financial or otherwise, concerning the material or methods used or the findings specified in this study.
ETHICAL STANDARDS
This study was approved by the Research Ethics Committee (No. KEXP63052).
FUNDING
There was no financial or material support for this study.



