-
PDF
- Split View
-
Views
-
Cite
Cite
Rodrigo Nascimento Pinheiro, Anderson Arantes Silvestrini, Gabriela El Haje Lobo Rocha, Heloisa Lima Heller, Camila de Carvalho Gallo Pereira, Rodrigo Guimarães Furtado, Radioisotope-guided laparoscopy: a case report and description of an alternative surgical technique, Journal of Surgical Case Reports, Volume 2022, Issue 3, March 2022, rjac069, https://doi.org/10.1093/jscr/rjac069
Close - Share Icon Share
Abstract
Prostate cancer is the second most frequent cancer type among men worldwide. With the development of Radiology and Nuclear Medicine technologies, early diagnosis is increasingly common, and the possibility of using new minimally invasive techniques increases. With a narrative review of the literature, this case report describes an alternative radioisotope-guided laparoscopy technique for tumors in the peritoneal cavity. There may be benefits in associating nuclear medicine techniques in the management of patients with non-palpable tumors that are difficult to locate in the peritoneal cavity, enabling the use of less invasive and safer surgical procedures for diagnosis, staging and treatment.
INTRODUCTION
Prostate cancer is the second most frequent cancer type among men worldwide [1, 2]. In Brazil, it is estimated 65 840 new cases for each year of the 2020–2022 triennium, corresponding to a risk of 62.95 new cases/100 000 men [3]. In the world, there are an estimated 1 100 000 cases annually [1].
The main risk factors for the disease are age over 50 years, first-degree family history, black ethnicity, androgen exposure and a high-fat diet. Most patients are asymptomatic; however, hematuria, obstructive and irritative symptoms may appear [1].
There is no definitive consensus on the best management of localized disease, but therapeutic options can be radical prostatectomy, external radiotherapy and active surveillance [4]. The indication of each therapy varies according to each case [1, 4].
For follow-up, total prostate-specific antigen (PSA) dosage is recommended every 6 months for 5 years and annually after that [2]. Therefore, it is considered a possible relapse if two consecutive dosages with values above 0.2 ng/mL, specifically after radical prostatectomy [2]. It is recommended to investigate by imaging exam to diagnose local recurrence or metastasis [2, 4].
With the development of Radiology and Nuclear Medicine technologies, the diagnosis of small lesions that are difficult to locate in the cavity at the time of surgery, becomes increasingly common. These small-sized tumors make minimally invasive approaches to diagnose or treat them more difficult or impossible. Within this context of approaching or re-approach cancer patients, radio-guided surgeries have become feasible and an option for management [5, 6]. Therefore, it may be necessary to establish a bridge between accurate diagnostic techniques such as positron emission computed tomography – PET/CT images with radioisotopes and minimally invasive surgeries, enabling the exact intraoperative location of small hypermetabolic tumoral lesions [7]. Using equipment such as a gamma probe, the surgeon can identify sentinel lymph nodes and preoperative occult lesions, confirm preoperative markings, and perform less invasive surgeries [5, 6].
This case report describes an alternative surgical technique, perhaps not yet represented in the literature and little used for locating small occult peritoneal tumors. In addition, the narrative review supports the benefit of associating Nuclear Medicine new technologies with the management of these patients.
CASE REPORT
M.J.A.P.P., 74 years old, male, white, mechanical engineer, presented in 2013 decreased urinary stream and altered rectal exam associated with a total PSA of 8.03 ng/mL and first-degree family history (father) of prostate cancer. Anatomopathological investigation confirmed usual acinar adenocarcinoma, Gleason 8 (4 + 4). The patient underwent a radical laparoscopic prostatectomy (performed by another team). Postoperatively, the total PSA was 0.08 ng/mL, and, due to involvement of the urethral surgical margin, local adjuvant radiation therapy was performed.
The disease remained stable until 2019, when the second consecutive increase in total PSA appeared, being 0.6 ng/mL on 11/26/2018 and 0.81 ng/mL on 01/07/2019. A Gallium-68 prostate-specific membrane antigen positron emission tomography (PET/CT-PSMA) imaging was performed, which showed a peritoneal nodule in the epigastric region, below the stomach and in front of the transverse colon, measuring 1.0 × 0.7 cm, with a significant increase in the expression of the specific antigen of prostate membrane – PSMA (SUV = 18.9). Due to the condition, suspicion of an implant was raised.
After a multidisciplinary team discussion with clinical oncology, surgical oncology and nuclear medicine followed by the patient’s free and informed consent, it was decided to perform diagnostic and therapeutic laparoscopy guided by the radioisotope. The lesion was marked with 0.5 mCi (18,5 MBq) of the radiopharmaceutical MAA-99mTc (macro aggregated human serum albumin) guided by computed tomography and confirmed by a SPECT image (single-photon emission computed tomography). This last image revealed a focal radiotracer concentration area in the upper left abdomen, measuring 3.5 and 5 cm deep from the skin, which did not change its appearance along with the scintigraphic evaluation (Fig. 1).
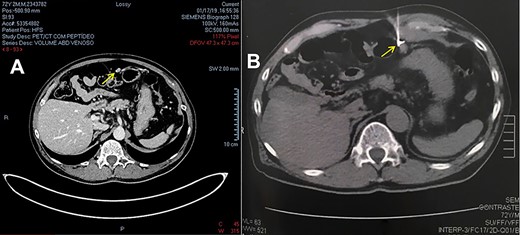
(A) CT image from PET-CT with PSMA-Gallium 68. (B) CT image of lesion marking with MAA-99mTc.
The following day, a gamma probe-guided laparoscopy was performed, and complete excision of the lesion was possible and uneventful. The lesion corresponded to two irregular fragments of fatty tissue, the largest being 3.0 × 1.3 × 0.5 cm, with an area of nodular aspect, covered by a thin capsule measuring 1.5 × 1.5 × 1.0 cm and, histological sections, exposed to a heterogeneous surface (Fig. 2). The histopathological result confirmed the lesion as a metastatic adenocarcinoma to peritoneal tissue. The immunohistochemistry showed a favorable immunophenotype for the prostate as the primary site showed positive PSMA and NKX3-1 antibodies and weakly positive MUC2. Currently, the patient is under follow-up, with no evidence of active disease.
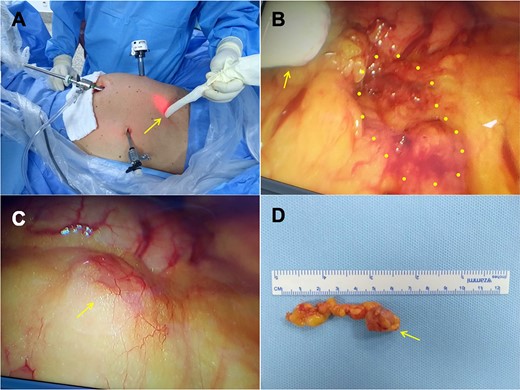
(A) The probe is localizing the lesion outside the abdominal cavity. (B) The arrow is pointing to the probe inside the abdominal cavity and the dotted circle shows the lesion. (C) Lesion seen by laparoscopy. (D) Removed lesion.
DISCUSSION
Radio-guided surgeries are a reality to several anatomical sites and organs, but they are still not well established for managing tumors of the peritoneal cavity. Sentinel lymph node techniques, for example, are well documented for breast cancer [8] and have also come to be suggested for other types of cancer [9]. These procedures usually consist of techniques using radioisotopes, followed by surgery with lymph node biopsy. They can result in the adequate staging of tumors and surgery with preservation of function [10].
Nowadays, the radioguided occult lesion localization (ROLL) is being used more frequently. In this technique, the lesion is marked with a radiopharmaceutical, such as technetium-labeled albumin macroaggregate, using an image-guided injection, followed by scintigraphy to analyze this marking. Intraoperatively, the lesion is identified with a gamma probe, and the marked tissue is removed [9, 11–14].
Radio-guided surgeries have some advantages over other techniques. Reduces surgical morbidity, can speed up the return to work. It can also make possible biopsies that cannot be performed by different approaches and facilitate the excision of the lesion by precise delimitation associated with the possibility of guided surgery during the procedure, avoiding location errors [15]. As these techniques improve, other clinical applications begin to emerge, making this approach an alternative tool for locating primary and metastatic tumors of different origins and various anatomical sites [9].
As noted, these techniques have the potential to establish themselves as a less invasive and safe alternative for diagnosis, staging and treatment that can also be used for peritoneal cavity tumors.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.



