-
PDF
- Split View
-
Views
-
Cite
Cite
James Foley, Ahmed Elamien, Brendan McCann, Morgan McMonagle, Axillary artery and brachial plexus injury secondary to blunt trauma, Journal of Surgical Case Reports, Volume 2021, Issue 3, March 2021, rjab068, https://doi.org/10.1093/jscr/rjab068
Close - Share Icon Share
Abstract
Rupture of the axillary artery after blunt trauma is an unusual injury, typically occurring in association with anterior dislocation of the shoulder or fracture-dislocation of the surgical head of the humerus. An associated blunt brachial plexus injury will typically accompany such an injury due to its intimate anatomical juxtaposition. We present the case of an elderly female, who presented after a fall from height, onto the outstretched arm, sustaining a combined axillary artery and brachial plexus blunt injury. The patient in this case recovered well post-operatively. The challenge in cases such as this is timely diagnosis and management of the vascular injury. The mechanism of injury combined with the presence or absence of hard signs of vascular injury should alert the clinician with rapid transition to investigation and management where appropriate, to minimise the warm ischaemic time to the upper limb and / or control of haemorrhage. Blunt injuries to the brachial plexus often require a prolonged period of time before accurate recovery can be prognosticated for.
INTRODUCTION
Blunt injury with rupture of the axillary artery is a rare traumatic finding, typically associated with an anterior dislocation of the shoulder or fracture of the surgical head of the humerus with anterior displacement of the fractured humeral shaft [1, 2]. An accompanying brachial plexus injury may occur due to concomitant forceful stretching of the nerve complex or as a result of direct trauma to the cords of the brachial plexus from the displaced bony fragment. Cases in the literature involving patients of advanced age typically involve shearing injuries to sclerotic, non-elastic vessels with concomitant stretching of the cords of the brachial plexus [3]. A significant injury to the axillary artery will often present with one or more of the hard signs of vascular injury (loss of pulse, active arterial (pulsatile) haemorrhage, expanding (pulsatile) haematoma and bruit / thrill over a haematoma) indicating ischaemia and / or active haemorrhage (Table 1). Emergent surgical exploration and repair is mandatory to prevent control bleeding and to prevent irreversible upper limb ischaemia. Typically, concomitant injury to the cords of the brachial plexus is managed in a delayed fashion [4]. We present the case of an elderly female who following a fall, sustained an axillary artery rupture combined with a brachial plexus injury and highlight the diagnostic and treatment challenges associated with concomitant injuries.
When hard signs of vascular injury are present, these are associated with a very high sensitivity for the presence of arterial trauma and mandates explorative surgery and management
| Hard Signs of Vascular Injury |
| • Loss of pulses (distal to the injury) |
| • Pulsatile (active arterial) haemorrhage |
| • Expanding / pulsatile haematoma |
| • Audible bruit / palpable thrill |
| Hard Signs of Vascular Injury |
| • Loss of pulses (distal to the injury) |
| • Pulsatile (active arterial) haemorrhage |
| • Expanding / pulsatile haematoma |
| • Audible bruit / palpable thrill |
When hard signs of vascular injury are present, these are associated with a very high sensitivity for the presence of arterial trauma and mandates explorative surgery and management
| Hard Signs of Vascular Injury |
| • Loss of pulses (distal to the injury) |
| • Pulsatile (active arterial) haemorrhage |
| • Expanding / pulsatile haematoma |
| • Audible bruit / palpable thrill |
| Hard Signs of Vascular Injury |
| • Loss of pulses (distal to the injury) |
| • Pulsatile (active arterial) haemorrhage |
| • Expanding / pulsatile haematoma |
| • Audible bruit / palpable thrill |
CASE REPORT
A 72-year-old female with a history of hypertension and hypercholesterolemia presented to the emergency department (ED) via ambulance. She sustained a fall from height (approx. One metre) while cleaning her ceiling at home falling onto her outstretched right arm. During the fall, she sustained blunt trauma to the right anterior chest (approximately the level of the right delto-pectoral groove) from the corner of a wooden window sill. There was no history of head injury, loss of consciousness and or neck trauma. She reported an immediate feeling of her right arm becoming ‘heavy’ and ‘difficult to move’, but delayed presentation for up to 12 hours, during which time her right arm became insensate, paralysed and cold.
On examination in the ED, there were no clinical signs of cervical spine injury, no obvious head injury and a Glasgow coma scale (GCS) was 15/15. Neurological examination of her right upper limb revealed a gross decrease in both motor and sensory function across dermatomes C5-T1 She had a cold hand and impalpable distal pulses (radial & brachial). The There was no gross bony tenderness in the right upper limb, but there was extensive soft tissue bruising at the level of the right delto-pectoral groove, which was non-pulsatile with no palpable thrill or audible bruit. The working diagnosis was a combined neurovascular injury secondary to a blunt trauma.
Plain radiographs of the right humerus and shoulder did not show any fracture or dislocation. A computed tomography (CT) scan with arterial phase angiography did not show and acute fracture of the cervical spine, but did reveal an abrupt cessation in flow in the right axillary artery secondary to traumatic occlusion of the vessel at the junction of segment 2 going into segment 3 with significant surrounding haematoma (Figs. 1 and 2).
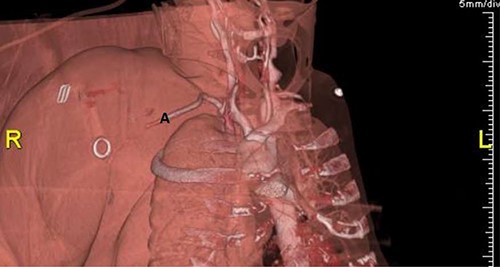
3D reconstruction of CT angiography showing abrupt cessation of flow beyond segment 1 of the right axillary artery (A).
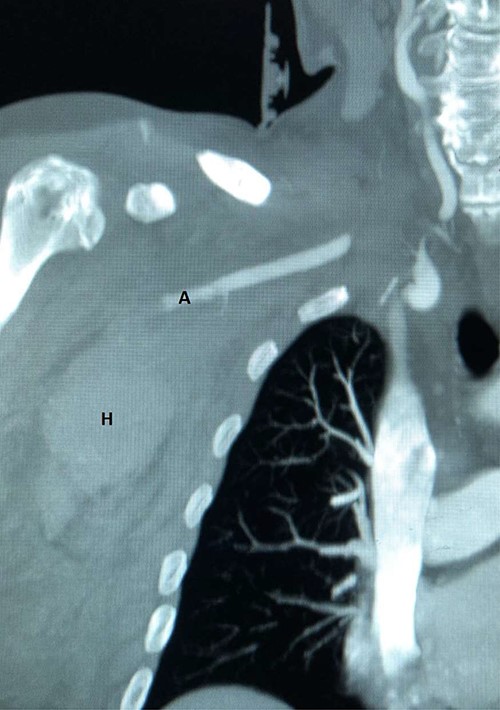
CT angiography reconstructed views demonstrating absent flow beyond segment 1 of the right axillary artery (A) with haematoma in the right axilla (H).
The patient was managed operatively by the vascular surgery team with emergent exploration. The axillary artery was dissected and controlled via an 8-10 cm infra-clavicular incision, extending into the right delto-pectoral groove. The fibres of the pectoralis major muscle were separated along the direction of the fibres to reveal the pectoralis minor muscle, which was subsequently divided to gain access to the subclavian vessels and accompanying cords of the brachial plexus (Figs. 3 and 4). There was extensive soft tissue distortion with a large haematoma, which was evacuated. The axillary artery was controlled proximally and distally with double looped vascular slings before clamping. Systemic heparanisation was not used. Examination of the vessel revealed injury to segments 2 and 3 with a full thickness laceration at the posterior aspect of segment 3The cords of the brachial plexus (posterior, median and lateral cords) were intact, but bruised, in particular the posterior cord, which was also displaced by the large haematoma from the posterior tear in the axillary artery. The injured vessel segment was resected, ensuring healthy vessel (in particular the intimal layer) remained. Empiric embolectomy of the distal vessel was performed using a size 3 and 4 Fogarty catheter and there was excellent in-flow and back flow to the now-resected injured segment. The distal portion was flushed copiously with heparanised saline.
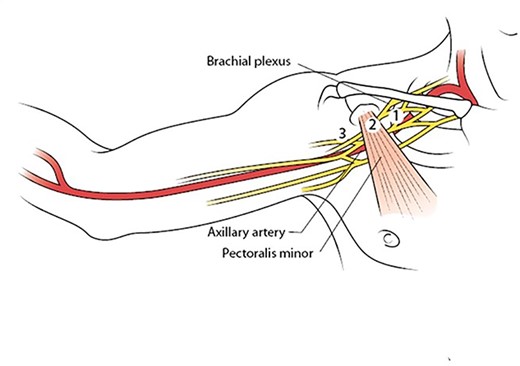
Illustration showing the three segments of the axillary artery as determined by the pectoralis minor muscle, which must be divided for complete exposure and safe control of the vessel. With permission from McMonagle MP. Vascular Trauma. In: Trauma: Code Red. Khan M, McMonagle MP, Nott D (Eds). CRC Press, Boca Raton, 2018.
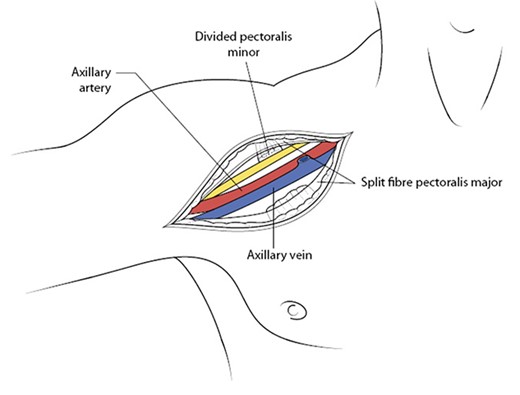
Once the pectoralis minor muscle has been divided, this brings into view the entire axillary artery along with the cords of the brachial plexus and its accompanying axillary vein (antero-posterior to the artery). With permission from McMonagle MP. Vascular Trauma. In: Trauma: Code Red. Khan M, McMonagle MP, Nott D (Eds). CRC Press, Boca Raton, 2018.
An interposition graft, using a 6 mm ring reinforced extended polytetrafluoroethylene (ePTFE) synthetic graft was anastomosed (5.0 Prolene) in situ, taking care to preserve the cords of the brachial plexus (Fig. 5).
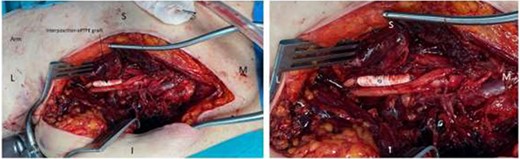
ePTFE synthetic graft (G) in situ. The medial cord of the brachial plexus is also seen as it becomes the median nerve (after receiving branch from lateral cord). The posterior cord is not visualised as it lies behind the graft. M = medial, L = lateral, S = superior, I = inferior.
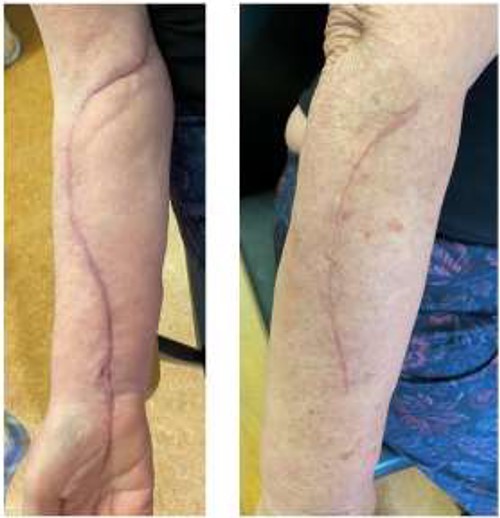
At the completion of the graft, limb perfusion was restored, with a return of the normal radial pulse. However, due to the prolonged ischaemic time prior to presentation, the risk of upper limb compartment syndrome was considered high and the patient underwent a forearm fasciotomy (volar and dorsal aspects) to mitigate against this (Fig. 6). These were closed primarily after a number of days and healed uneventfully (Fig. 7). The right upper limb remained well-perfused during the post-operative period although wrist-drop persisted for several months secondary to bruising of the posterior cord of the brachial plexus. This bruising of the cords was confirmed on subsequent follow up MRI scan. However, the cords were intact and the patients neurological symptoms and signs improved over the coming months with physiotherapy.
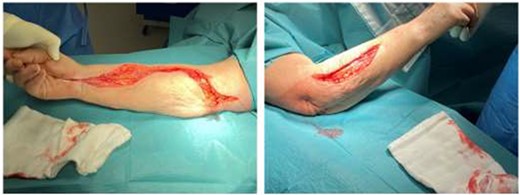
Forearm fasciotomy involving both volar (flexure compartment) and dorsal (extensor compartment) incisions. The volar incision releases the flexure compartment (the flexure muscles are more important for hand grip than the extensor muscles compartment). This is performed by starting at the medial aspect of the humerus (approximately where the brachial artery would be dissected ordinarily) and carried across the olecranon fossa as a transverse incision (to prevent a skin contracture) and then crossing the volar aspect of the forearm in a wavy pattern, crossing transversely at least once (to prevent a skin contracture). The incision is further carried across the wrist (and the flexure retinaculum is divided) taking care not to injure the median nerve here, and into the palmer fascia for about 2-3 cm (often following the line of the thenar eminence). The dorsal incision is a straight or slightly semi-lunar incision to release the extensor compartment.
DISCUSSION
The incidence of a combined axillary artery and brachial plexus injury in the literature is reported as 27–44% [3]. The axillary artery is the most frequently affected artery vessel after shoulder trauma, typically involving the third segment of the vessel (ie: from the lateral border of pectoralis minor muscle to the lower border of teres major muscle), due to the relative tethering of the vessel at the lateral margin of the former muscle, especially during abduction and external rotation of the shoulder joint [5].
The warm ischaemia time after arterial occlusion has been reported in the literature as 4 hours for proximal lesions (ie: proximal to the profunda brachii branch), but up to 12 hours distal to this [3, 6]. Thus, timely diagnosis and management with clinical exam (ie: the presence or absence of hard signs of vascular injury) with or without CT angiography (where appropriate) [4]. Although the upper limb is more resistant to warm ischaemia compared with the lower limb, a missed opportunity to reperfuse the upper limb can lead to a greater disability, especially when the dominant arm is involved (eg: chronic ischaemic neuropathy, chronic ischaemic neuritis, ischaemic contracture, etc).
Blunt brachial plexus trauma may result in a neuropraxia or axonotmesis, but rarely requires any operative management, often improving within 3–6 months [4, 5]. However, cord laceration should be operatively repaired, with associated superior outcomes when directly repaired early rather than late as the denervated muscle becomes fibrotic and functional outcome may remain poor [2]. Torn cords, if found at the index procedure in the haemodynamically unwell patient who has lost significant volumes of blood, will not withstand a prolonged nerve repair operation. In this scenario, the torn nerve endings should be marked by the surgeon (eg: liga clips) and a delayed repair performed when more appropriate. Various neurological investigations are typically unhelpful (eg: nerve conduction and electromyography) as they may be misleading during the acute phase of presentation (eg: absence of decreased conduction for 7–10 days) [2, 7]. Therefore, a high index of suspicion should alert the surgeon when abnormal neurological findings are found on examination.
In conclusion, timely diagnosis of an arterial injury includes close clinical examination for hard signs of vascular injury with or without CT angiography for the haemodynamically well patient. Surgeons must familiarise themselves with operative management of axillary artery injuries, in the absence of further diagnostic studies, due to the higher prevalence of shock and greater mortality when an actively bleeding arterial injury is present. In the haemodynamically well patient, the capillary refill may be normal, even in the presence of hard signs of vascular injury due to collateral blood supply around the shoulder girdle. An associated nerve injury can be more challenging to recognise as the findings may also be aggravated by the vascular compromise. The mechanism of injury in addition to the clinical findings should alert the clinician to the possibly of such injuries. If a vascular injury is suspected, early diagnosis with prompt surgical revascularisation is associated with superior outcomes. Consideration must be given to the possibility of a concomitant brachial nerve cord injury at the time of surgical exploration and managed accordingly to mitigate against a long term functional disability.
AUTHORS’ CONTRIBUTIONS
JF and AE wrote the case and edited the manuscript. BM and MM supervised the writing of the manuscript.
PATIENT CONSENT
Consent for this case report was provided by the patient and documented in the hospital chart.
CONFLICT OF INTEREST STATEMENT
There are no conflicts of interest to report in this case.
FUNDING
There no financial or funding disclosures.
References
- ischemia
- embolectomy
- axilla
- axillary artery
- brachial plexus
- humerus
- rupture
- shoulder dislocations
- surgical procedures, operative
- time factors
- wounds and injuries
- nonpenetrating wounds
- arm
- diagnosis
- fracture dislocation
- vascular injuries
- older adult
- brachial plexus injuries
- hemorrhage control
- mechanism of injury



