-
PDF
- Split View
-
Views
-
Cite
Cite
Pantelis Diamantopoulos, Panagiotis Gouveris, Stavroula Diamantopoulou, Lilika Tseloni, Evdokia Arkoumani, Niki Arnogiannaki, Spiridon Stavrianos, Collision tumor of malignant tumors of the skin: dermal squamomelanocytic tumor coexisting with basal cell carcinoma—a rare case, Journal of Surgical Case Reports, Volume 2021, Issue 12, December 2021, rjab560, https://doi.org/10.1093/jscr/rjab560
Close - Share Icon Share
Abstract
Collision tumors are neoplasms coexisting in the some anatomical area. The most common combination is melanocytic nevus with basal cell carcinoma. Melanocytic nevus with basal cell carcinoma constitutes the most common cutaneous combination. Co-existence of two malignant neoplasms is extremely rare. We describe the case of a 69-year-old man who was admitted to our hospital with a nodular mass on the back. We performed an excisional biopsy that revealed collision tumor, consisting of basal cell carcinoma along with mixed melanosquamous carcinoma. Subsequently, wide excision with sentinel node biopsy was performed. The sentinel node was negative. The patient did not receive any ongologic therapy.
INTRODUCTION
Co-existence of two histologically distinct tumors in the same anatomic location is called collision tumor. Although rare, the combination of two malignant skin tumors such as melanoma and basal cell carcinoma (BCC) has been reported to the literature [1]. Moreover, the coexistence of basal cell with combined melanosquamous carcinoma has never been reported before.
The first reported case of melanoma with BCC was presented by Kao in 1983 [2].
The immunohistochemistry is usually very useful in defining the tumors [3]. There is no mixing or interaction between the different tumor cells.
CASE REPORT
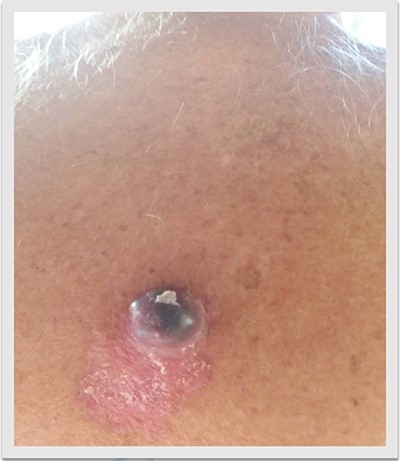
A 69-year-old man was admitted to our hospital with a nodular skin tumor of the back (Fig. 1). The patient had no ongologic history. We performed excisional biopsy that revealed collision tumor. The collision tumor consisted of mixed melanosquamous tumor (dermal squamomelanocytic tumor) together with BCC (Fig. 2). The BCC was superficial spreading. The mixed element contained squamous cell carcinoma of well/moderately differentiated and a neoplasm with melanotic characteristics, which due to cell atypia, presence of mitoses and high index of cell proliferation (Ki67: 80%) was described as melanoma (Fig. 3). Diagnosis of the melanotic and the squamous element was confirmed with immunohistochemistry (Figs 4 and 5).
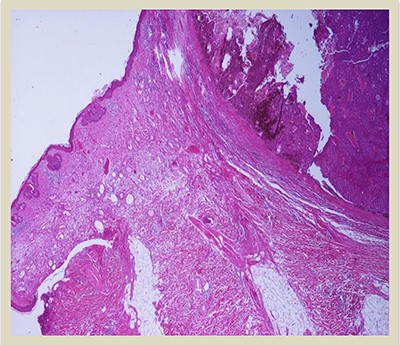
H–E × 2: Collision tumor: in the right side of the photo is shown the dermal squamomelanocytic tumor component and in the left side the superficial basal cell carcinoma (H–E stain ×2 magnification).
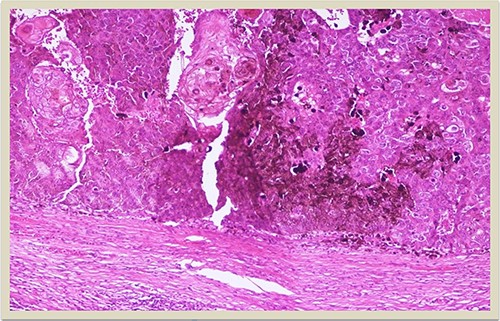
H–E × 10: Higher magnification of the dermal squamomelanocytic tumor showing admixed malignant malanocytes and keratinizing squamous cell carcinoma cells (H–E stain ×10 magnification).
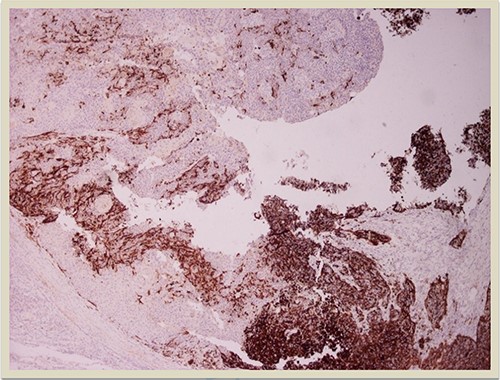
HMB45 × 4: HMB45 immunostain highlights the malignant melanocytic component ( ×10 magnification).
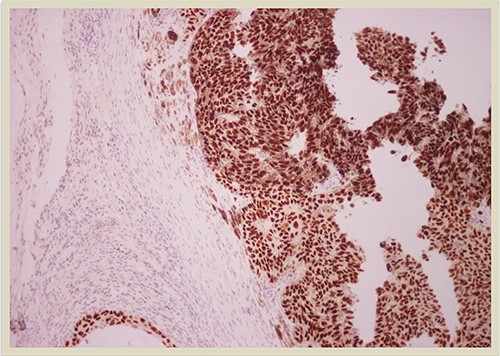
p63 × 10: p63 immunostain confirms the presence of squamous cell carcinoma component (×10 magnification).
The melanosquamous tumor did not involve epidermis, therefore there was not result about Breslow thickness in the histologic report. Because of the presence of the melanoma sentinel lymph node biopsy was performed (Fig. 6). The excised lymph node showed no evidence of metastasis.
Molecular analysis did not reveal serine/threonine-protein kinase (BRAF) mutation. The patient underwent brain, chest and abdomen computed tomography (CT) and no metastases were detected. The positron emission tomography-CT was also negative. Because of the negative sentinel lymph node, the oncologic board decided that no oncologic therapy was necessary. Since then the patient has been intensively followed-up and remains disease free.
DISCUSSION
Collision tumors containing melanoma and other types of skin cancer have been described in the literature before. However the most common combination is in situ melanoma along with BCC [4]. The presence of infiltrative melanoma in a collision tumor is relatively uncommon. To our knowledge the presence of a mixed melanotic tumor in a collision has never been described in the literature. In our case the collision neoplasm consisted of a mixed melanosquamous carcinoma along with BCC.
Pathogenesis of collision neoplasms is unknown and a matter of speculation. Chronic exposure to sunlight is a critical factor for developing melanoma and other types of skin cancer such as BCC and squamous cell carcinoma (Scc). In our patient, the development of a collision tumor in an area of photodamage can reasonably be explained.
Biological behavior of a collision tumor depends directly on the most aggressive neoplasm, which is melanoma in our case. That’s why we treated the lesion with wide local excision and sentinel lymph node biopsy. The sentinel lymph node was negative for metastatic disease so we did not have to perform lymph node dissection of the entire nodal basin.
Histologically the collision tumor involves two different and distinct entities. There is no interaction between the tumor cells of the two different neoplasms. The paradox in our case is that the melanotic cells were admixed with squamous cells. The histopathologic findings of the combined tumor were confirmed with immunohistochemistry. Squamous element was confirmed from the positivity of the stains: AE1/AE3, EMA and CK5. Melanotic element was also confirmed with the following stains: Vimentin, S-100, MART-1(Melan-A) and HMB45. Histologic findings and immunohistochemical stains confirmed the combined melanosquamous neoplasm in the collision tumor, which does not seem to involve epidermis. Reviewing the literature, we did not find any other case of a combined neoplasm in a collision formation.
The intermingling of two neoplasms in the same cutaneous entity is extremely rare [5]. A squamomelanotic tumor (SMT) combines histological features of squamous cell carcinoma and malignant melanoma. There are only 18 cases of SMT reported in the literature [5]. In our case, the tumor involves admixture of melanocytic and squamous cells.
On the contrary, the collision tumor does not involve mixing of the components, but distinctly separate melanocytic and epithelial cells. However, what is paradox in our case in our case is that the melanoma component did not involve epidermis. That’s why there was no result about Breslow thickness in the histology report. Breslow thickness represents the depth of melanoma infiltration, it is typically measured from the granular layer of the epidermis down to the deepest point of invasion, and dictates the recommended surgical margins when we perform wide excision. Safety margins vary between 1 and 2 cm, according to the Breslow thickness. We used 2 cm safety margins in our wide excision.
BCC carcinoma needs excisional biopsy with 3–4 mm surgical margin. In our case, because of the high suspicion of melanotic element we performed excisional biopsy of narrow margins in order not to distort the lymphatic drainage and be able to perform an accurate lymphatic mapping and sentinel lymph node biopsy for staging.
After the completion of surgical management in our clinic, the ongologists decided not to give additional therapy because sentinel lymph node was negative there was no disease detected by the imaging studies. Chemotherapy and radiotherapy have nowadays little use in the management of melanoma. Actually, checkpoint inhibitor immunotherapy with nivolumab or pembrolizumab, and targeted therapy with the combination of a B-RAF inhibitor and mitogen-activated protein kinase (MEK) inhibitor constitute the gold standard of adjuvant treatment in stage III melanoma. Regarding BCC, when it is excised with negative surgical margins is considered a cured disease [6] Recurrent BCCs are harder to treat, with a tendency for new recurrences.
Patients with collision tumors involving melanoma need intensive follow-up for 5–10 years for detection of possible local or distant recurrences. Patients with melanoma history have 10% probability of developing melanoma in 5 years [7].
We present our case because of the paradox co-existence of combined melanosquamous carcinoma along with BCC as a collision tumor. Melanosquamous carcinoma is a rare entity on it’s own, and it is even rarer as a part of a collision tumor. We highlight the role of immunohistochemistry on distinguishing the tumor cells and validating the diagnosis.
CONFLICT OF INTEREST STATEMENT
None declared.



