-
PDF
- Split View
-
Views
-
Cite
Cite
Kevin J Fuentes-Calvo, Luis F Arias-Ruiz, Irving Fuentes-Calvo, Angélica D Hidalgo-Maldonado, María F Aparicio-Sosa, Edson Escandón-Villalobos, Luis O González-Alcocer, Oscar Aguilar-Ruiz, Rita Dorantes-Heredia, Gonzalo Torres-Villalobos, Complicated appendicitis as a rare presentation of Burkitt’s lymphoma: a case report, Journal of Surgical Case Reports, Volume 2025, Issue 7, July 2025, rjaf585, https://doi.org/10.1093/jscr/rjaf585
Close - Share Icon Share
Abstract
Burkitt lymphoma (BL) is an aggressive B-cell non-Hodgkin lymphoma that rarely involves the appendix and may mimic acute appendicitis, complicating preoperative diagnosis. In the context of nonoperative management for appendicitis, such malignancies risk being overlooked. A 32-year-old immunocompetent male presented with right upper quadrant pain, leukocytosis, cholestatic liver profile, and a hepatic lesion on imaging. Positron emission tomography-computed tomography (PET-CT) showed intense fluorodeoxyglucose uptake in the colon, liver, and peritoneum. Laparoscopy revealed an enlarged appendix (15 × 3 cm) with abscess; appendectomy was performed. Histopathology confirmed BL with a ‘starry sky’ pattern and Ki-67 of 95%. Appendiceal BL, though rare, should be suspected in atypical or complicated appendicitis, even without classic risk factors. Histopathological examination of appendectomy specimens is crucial. Surgery, even when not oncologic in intention, may lead to early cancer detection and timely systemic treatment, improving outcomes.
Introduction
Acute appendicitis is a frequent surgical emergency, usually benign, but rare neoplastic causes like Burkitt lymphoma (BL) must be considered [1]. This aggressive B-cell lymphoma may mimic uncomplicated appendicitis, complicating diagnosis [1].
With increasing use of nonoperative management, the risk of overlooking incidental malignancies has grown, underscoring the value of appendectomy in atypical cases [1, 2]. BL, often seen in young or immunocompromised patients, frequently involves the gastrointestinal tract, including the appendix [3–5].
These tumors can present as an acute abdomen, but preoperative detection is difficult. Thus, histopathological analysis remains essential for diagnosis [4, 6, 7]. Prompt surgical evaluation and multidisciplinary treatment are critical, given the tumor’s rapid progression and poor prognosis without early therapy [1, 5].
Methods
The physical and electronic medical records of a patient treated at Medica Sur Hospital by G.T.V. and the surgery department were reviewed. Informed consent was obtained from the patient or their legal representative. The case report was prepared in accordance with the CARE Guidelines [8].
Case presentation
A 32-year-old male with no relevant medical history presented with a 5-day history of spasmodic right upper quadrant abdominal pain radiating to the right scapula. Physical exam revealed tenderness in the right upper quadrant and a positive Murphy’s sign. Labs showed leukocytosis (15.2 × 109/L) with neutrophilia, elevated C-reactive protein (447 mg/L), and cholestatic liver function tests.
Abdominal ultrasound showed a hypoechoic, avascular lesion in hepatic segment VI (31 × 37 × 26 mm) without Doppler flow. Triphasic computed tomography (CT) confirmed an irregular, heterogeneous lesion in the same area. Given the suspicion of a neoplastic or infectious hepatic lesion, a multidisciplinary team (surgery, gastroenterology, radiology) was convened for further evaluation and treatment planning.
Colonoscopy demonstrated a right-sided subepithelial colonic lesion measuring ⁓30 mm (Fig. 1). Positron emission tomography-computed tomography (PET-CT) revealed circumferential thickening of the ascending colon with intense fluorodeoxyglucose (FDG) uptake (SUVmax 20.0), along with multiple peritoneal implants, mesenteric, perigastric, and right subdiaphragmatic lymphadenopathy, as well as hypermetabolism in the previously identified hepatic lesion (SUVmax 19.8), findings highly suggestive of neoplastic dissemination (Fig. 2).
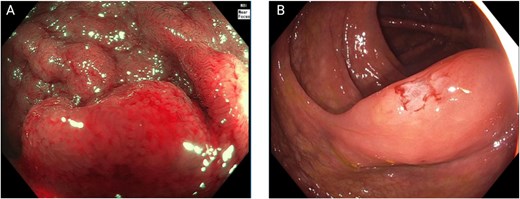
Colonoscopic findings. (A) Near-focus narrow-band imaging showing a prominent, nodular subepithelial lesion measuring ⁓30 mm, with disrupted mucosal architecture, erythema, and loss of surface vascular pattern—features suggestive of acute mucosal infiltration. (B) Conventional white-light endoscopy demonstrating smooth, elevated mucosa with focal congestion and superficial linear disruption, corresponding to the area of the subepithelial lesion.
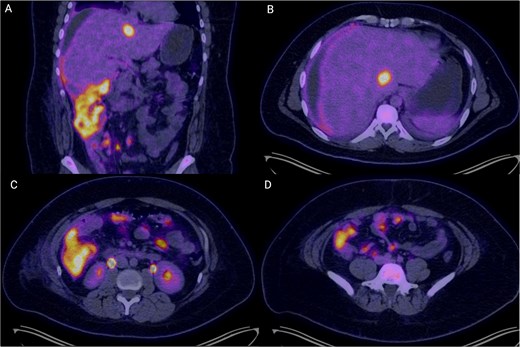
PET-CT images demonstrating extensive hypermetabolic abdominal disease. (A) Coronal image showing circumferential mural thickening of the right colon with intense FDG uptake (SUVmax 20.0), suggestive of transmural neoplastic infiltration, alongside multiple peritoneal implants and mesenteric hypermetabolism. (B) Axial slice at the hepatic level revealing a hypodense, hypermetabolic lesion in segment VI of the liver (SUVmax 19.8), consistent with a secondary metastatic deposit. (C) Axial mid-abdominal view showing abnormal FDG uptake in mesenteric and pericolonic regions, with nodular foci consistent with lymphadenopathy and peritoneal seeding. (D) The inferior axial section depicts multiple hypermetabolic foci along the peritoneum, indicative of diffuse peritoneal involvement. These findings are consistent with metabolically active, disseminated abdominal disease in keeping with a diagnosis of high-grade lymphoma.
Due to clinical and radiologic findings suggestive of advanced neoplasia with intra-abdominal infection, a diagnostic laparoscopy was performed. It revealed an enlarged, firm appendix (15 × 3 cm) with dense inflammatory phlegmon and a 30 mL periapendicular abscess with fibrinopurulent material. Laparoscopic drainage and appendectomy were performed without complications.
The surgical piece was sent to the pathology department, where it was observed the serosal surface of the appendix appeared brown with widespread fibrinopurulent plaques. Cross-sections revealed a white, soft tumor diffusely infiltrating the entire appendiceal wall and mesoappendix (Fig. 3).
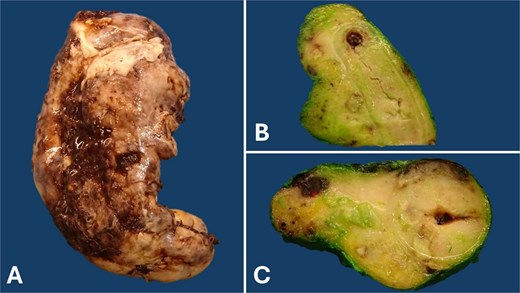
Gross pathology of the appendix: The serosal surface of the appendix appears brown, with fibrinopurulent plaques (A). Longitudinal (B) and cross (C) sections reveal obliteration of the appendiceal lumen by a diffusely growing, bulging, soft, and white tumor involving the entire appendiceal wall and mesoappendix. This appearance is often described as a ‘fish flesh’ appearance.
Histologically, the tumor was composed of monomorphic medium-sized lymphocytes with round nuclei, dispersed and clumped chromatin, basophilic nucleoli, and squared-off cell membranes. These lymphocytes are altered with scattered tingible body macrophages, creating a characteristic ‘starry sky’ pattern (Fig. 4).
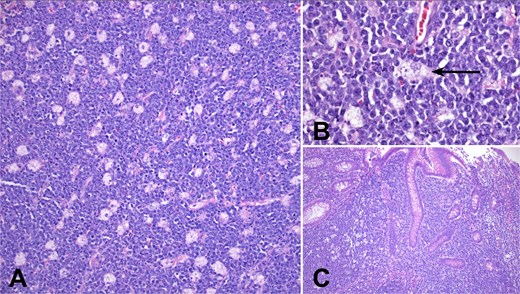
Microscopic tumor morphology: (A) In sections stained with H&E, the tumor shows a starry sky pattern composed of monomorphic medium-sized neoplastic lymphocytes and clear tingible body macrophages (100×). (B) Lymphocytes make the sky while tingible body macrophages (arrow) the sky of the starry sky pattern. Neoplastic lymphocytes exhibit a round nucleus, basophilic nucleoli, basophilic cytoplasm, and squared-off membranes (200×). (C) The lymphoma invades the appendiceal mucosa (100×).
In the immunohistochemistry analysis, neoplastic lymphocytes tested positive for CD20, CD10, BCL6, and c-MYC. CD5, BCL2, and TdT were negative in neoplastic cells. The proliferation index was 95%, measured with MIB1. In situ hybridization for Epstein–Barr virus-encoded RNA (EBER) was negative (Fig. 5).
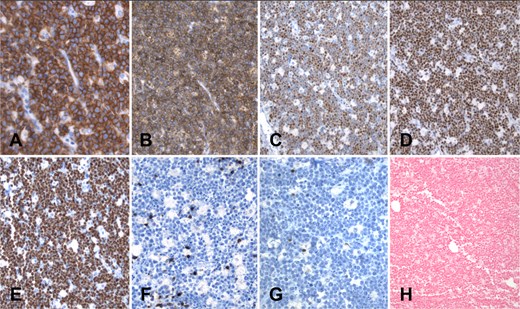
Immunohistochemistry panel (A–G) and in situ hybridization (H): Neoplastic cells exhibit diffuse membrane positivity for CD20 (A) and CD10 (B). Nuclear positivity is observed with the markers BCL6 (C) and c-MYC (D). Proliferation index measured with Ki67 (MIB1) is close to 100%; only scarce lymphocytes and macrophages are negative for MIB1 (E). CD5 (F) is negative in lymphoma cells, and only a few scattered reactive T cells are positive. BCL2 (G) is negative. Finally, in situ hybridization for Epstein–Barr virus, EBER (H), is completely negative in this case.
Histology and immunophenotype confirmed BL of the appendix with transmural involvement. The patient had a favorable postoperative course and was referred to oncology for further workup and initiation of systemic treatment.
Discussion
BL is a highly aggressive B-cell non-Hodgkin lymphoma and is considered the fastest-growing human tumor [9]. Its incidence is notably high, with an aggressive clinical course in immunosuppressed patients in non-endemic areas, particularly when associated with human immunodeficiency virus (HIV) infection. Clinically, patients with BL typically do not present with B symptoms (fever, chills, night sweats, and weight loss) that are characteristic of early stage non-Hodgkin lymphoma. This is attributed to the transformation of these cells, which compromises host defenses and develops mechanisms to evade immune surveillance [3].
Patients with rapidly growing intra-abdominal extranodal BL often present with symptoms of intestinal obstruction, intussusception, or appendicitis [4]. Although non-Hodgkin lymphoma is the most common malignant intestinal tumor in children over 5 years of age, BL presenting as appendicitis is rare [5].
If intra-abdominal BL is suspected, especially in endemic regions, preoperative imaging—either ultrasonography in expert hands or CT—is an effective tools for diagnosing the malignant nature of the tumor [6, 7]. Furthermore, histopathological examination following an appendectomy for apparent appendicitis is mandatory, as it may reveal ileocecal BL.
This study reports a condition that has been described in the literature primarily in patients with associated risk factors, such as residence in malaria-endemic areas, family history, or association with Epstein–Barr virus (EBV) or HIV infection. However, in our experience, the patient did not present any of these aforementioned risk factors, making this case even more unusual.
Currently, there are no clinical guidelines for its treatment; most evidence comes from case reports, series, and expert consensus based on clinical experience. Early identification of BL is crucial, as it is highly aggressive and rapidly metastasizes. Postoperative hematology-oncology follow-up is essential for comprehensive care.
A literature review revealed that, to the best of our knowledge, this is the first reported case in Mexico. Therefore, it represents a relevant contribution to the understanding of the clinical and surgical approach to this disease, offering a foundation for the development of a future learning curve in healthcare centers with similar populations and clinical presentations. This could significantly improve patient outcomes in such settings (Table 1).
| Authors . | Title . | Year . | Journal . | Country . | |
|---|---|---|---|---|---|
| 1 | Yahya HA, Kumar V, Lam JT | Burkitt Lymphoma of the Appendix Presenting With Acute Appendicitis and Acute Kidney Injury: A Case Report and Review of Literature | 2022 | Cureus | USA |
| 2 | Shaw T, Cockrell H, Panchal R, Abraham A, Sawaya D | Burkitt Lymphoma Presenting as Perforated Appendicitis | 2022 | Am Surg | USA |
| 3 | Shahmanyan D, Saway B, Palmerton H, Rudderow JS, Reed CM, Wattsman TA, Faulks ER, Collier BR, Budin RE, Hamill ME | Burkitt-type lymphoma incidentally found as the cause of acute appendicitis: a case report and review of literature | 2021 | Surg Case Rep | USA |
| 4 | Dashottar S, Sunita BS, Singh RK, Rana V, Suhag V, Singh AK | A case series of unusual presentations of Burkitt’s lymphoma | 2020 | J Cancer Res Ther | India |
| 5 | Mimery AH, Jabbour J, Sykes B, MacDermid E, Al-Askari M, De Clercq S | Burkitt Leukemia Presenting as Acute Appendicitis: A Case Report and Literature Review | 2020 | Am J Case Rep | USA |
| 6 | Nam S, Kang J, Choi SE, Kim YR, Baik SH, Sohn SK | Xanthogranulomatous Appendicitis Mimicking Residual Burkitt’s Lymphoma After Chemotherapy | 2016 | Ann Coloproctol | South Korea |
| 7 | Sangma MM, Dasiah SD, Ashok AJ | Ileo-Colic Burkitt Lymphoma in a Young Adult Female- A Case Report | 2016 | J Clin Diagn Res | India |
| 8 | Vrancx S, Van de Sande J, Vanclooster P, de Gheldere C, Van de Velde A | Burkitt Lymphoma Mimicking an Acute Appendicitis in a 17 Years Old Boy: a Case Report | 2015 | Acta Chir Belg | Belgium |
| 9 | Weledji EP, Ngowe MN, Abba JS | Burkitt’s lymphoma masquerading as appendicitis—two case reports and review of the literature | 2014 | World J Surg Oncol | Cameroon |
| 10 | Bhardwaj N, Bains SK, Ortonowski G, Murphy P | A case of Burkitt’s lymphoma presenting as suspected acute appendicitis | 2010 | Afr J Paediatr Surg | India |
| 11 | Wang SM, Huang FC, Wu CH, Ko SF, Lee SY, Hsiao CC | Ileocecal Burkitt’s lymphoma presenting as ileocolic intussusception with appendiceal invagination and acute appendicitis | 2010 | J Formos Med Assoc | Taiwan |
| 13 | Caine YG, Peylan-Ramu N, Livoff AF, Schiller M | Primary Burkitt’s lymphoma of the appendix | 1990 | Z Kinderchir | Germany |
| 14 | Ghani SA, Syed N, Tan PE | A rare cause of acute appendicitis: Burkitt’s lymphoma of the appendix | 1984 | Med J Malaysia | Malaysia |
| 15 | Nanji AA, Anderson FH | Burkitt’s lymphoma with acute appendicitis | 1983 | Arch Surg | Canada |
| 16 | Sin IC, Ling ET, Prentice RS | Burkitt’s lymphoma of the appendix: report of two cases | 1980 | Hum Pathol | USA |
| Authors . | Title . | Year . | Journal . | Country . | |
|---|---|---|---|---|---|
| 1 | Yahya HA, Kumar V, Lam JT | Burkitt Lymphoma of the Appendix Presenting With Acute Appendicitis and Acute Kidney Injury: A Case Report and Review of Literature | 2022 | Cureus | USA |
| 2 | Shaw T, Cockrell H, Panchal R, Abraham A, Sawaya D | Burkitt Lymphoma Presenting as Perforated Appendicitis | 2022 | Am Surg | USA |
| 3 | Shahmanyan D, Saway B, Palmerton H, Rudderow JS, Reed CM, Wattsman TA, Faulks ER, Collier BR, Budin RE, Hamill ME | Burkitt-type lymphoma incidentally found as the cause of acute appendicitis: a case report and review of literature | 2021 | Surg Case Rep | USA |
| 4 | Dashottar S, Sunita BS, Singh RK, Rana V, Suhag V, Singh AK | A case series of unusual presentations of Burkitt’s lymphoma | 2020 | J Cancer Res Ther | India |
| 5 | Mimery AH, Jabbour J, Sykes B, MacDermid E, Al-Askari M, De Clercq S | Burkitt Leukemia Presenting as Acute Appendicitis: A Case Report and Literature Review | 2020 | Am J Case Rep | USA |
| 6 | Nam S, Kang J, Choi SE, Kim YR, Baik SH, Sohn SK | Xanthogranulomatous Appendicitis Mimicking Residual Burkitt’s Lymphoma After Chemotherapy | 2016 | Ann Coloproctol | South Korea |
| 7 | Sangma MM, Dasiah SD, Ashok AJ | Ileo-Colic Burkitt Lymphoma in a Young Adult Female- A Case Report | 2016 | J Clin Diagn Res | India |
| 8 | Vrancx S, Van de Sande J, Vanclooster P, de Gheldere C, Van de Velde A | Burkitt Lymphoma Mimicking an Acute Appendicitis in a 17 Years Old Boy: a Case Report | 2015 | Acta Chir Belg | Belgium |
| 9 | Weledji EP, Ngowe MN, Abba JS | Burkitt’s lymphoma masquerading as appendicitis—two case reports and review of the literature | 2014 | World J Surg Oncol | Cameroon |
| 10 | Bhardwaj N, Bains SK, Ortonowski G, Murphy P | A case of Burkitt’s lymphoma presenting as suspected acute appendicitis | 2010 | Afr J Paediatr Surg | India |
| 11 | Wang SM, Huang FC, Wu CH, Ko SF, Lee SY, Hsiao CC | Ileocecal Burkitt’s lymphoma presenting as ileocolic intussusception with appendiceal invagination and acute appendicitis | 2010 | J Formos Med Assoc | Taiwan |
| 13 | Caine YG, Peylan-Ramu N, Livoff AF, Schiller M | Primary Burkitt’s lymphoma of the appendix | 1990 | Z Kinderchir | Germany |
| 14 | Ghani SA, Syed N, Tan PE | A rare cause of acute appendicitis: Burkitt’s lymphoma of the appendix | 1984 | Med J Malaysia | Malaysia |
| 15 | Nanji AA, Anderson FH | Burkitt’s lymphoma with acute appendicitis | 1983 | Arch Surg | Canada |
| 16 | Sin IC, Ling ET, Prentice RS | Burkitt’s lymphoma of the appendix: report of two cases | 1980 | Hum Pathol | USA |
The search criteria (‘Burkitt lymphoma’ [All Fields]) AND (‘appendicitis’ [All Fields]) were applied in databases such as PubMed, Scopus, and Google Scholar. Articles that were not case reports or case series were excluded, such as those without full-text availability in English or Spanish or those not indexed in the databases above.
| Authors . | Title . | Year . | Journal . | Country . | |
|---|---|---|---|---|---|
| 1 | Yahya HA, Kumar V, Lam JT | Burkitt Lymphoma of the Appendix Presenting With Acute Appendicitis and Acute Kidney Injury: A Case Report and Review of Literature | 2022 | Cureus | USA |
| 2 | Shaw T, Cockrell H, Panchal R, Abraham A, Sawaya D | Burkitt Lymphoma Presenting as Perforated Appendicitis | 2022 | Am Surg | USA |
| 3 | Shahmanyan D, Saway B, Palmerton H, Rudderow JS, Reed CM, Wattsman TA, Faulks ER, Collier BR, Budin RE, Hamill ME | Burkitt-type lymphoma incidentally found as the cause of acute appendicitis: a case report and review of literature | 2021 | Surg Case Rep | USA |
| 4 | Dashottar S, Sunita BS, Singh RK, Rana V, Suhag V, Singh AK | A case series of unusual presentations of Burkitt’s lymphoma | 2020 | J Cancer Res Ther | India |
| 5 | Mimery AH, Jabbour J, Sykes B, MacDermid E, Al-Askari M, De Clercq S | Burkitt Leukemia Presenting as Acute Appendicitis: A Case Report and Literature Review | 2020 | Am J Case Rep | USA |
| 6 | Nam S, Kang J, Choi SE, Kim YR, Baik SH, Sohn SK | Xanthogranulomatous Appendicitis Mimicking Residual Burkitt’s Lymphoma After Chemotherapy | 2016 | Ann Coloproctol | South Korea |
| 7 | Sangma MM, Dasiah SD, Ashok AJ | Ileo-Colic Burkitt Lymphoma in a Young Adult Female- A Case Report | 2016 | J Clin Diagn Res | India |
| 8 | Vrancx S, Van de Sande J, Vanclooster P, de Gheldere C, Van de Velde A | Burkitt Lymphoma Mimicking an Acute Appendicitis in a 17 Years Old Boy: a Case Report | 2015 | Acta Chir Belg | Belgium |
| 9 | Weledji EP, Ngowe MN, Abba JS | Burkitt’s lymphoma masquerading as appendicitis—two case reports and review of the literature | 2014 | World J Surg Oncol | Cameroon |
| 10 | Bhardwaj N, Bains SK, Ortonowski G, Murphy P | A case of Burkitt’s lymphoma presenting as suspected acute appendicitis | 2010 | Afr J Paediatr Surg | India |
| 11 | Wang SM, Huang FC, Wu CH, Ko SF, Lee SY, Hsiao CC | Ileocecal Burkitt’s lymphoma presenting as ileocolic intussusception with appendiceal invagination and acute appendicitis | 2010 | J Formos Med Assoc | Taiwan |
| 13 | Caine YG, Peylan-Ramu N, Livoff AF, Schiller M | Primary Burkitt’s lymphoma of the appendix | 1990 | Z Kinderchir | Germany |
| 14 | Ghani SA, Syed N, Tan PE | A rare cause of acute appendicitis: Burkitt’s lymphoma of the appendix | 1984 | Med J Malaysia | Malaysia |
| 15 | Nanji AA, Anderson FH | Burkitt’s lymphoma with acute appendicitis | 1983 | Arch Surg | Canada |
| 16 | Sin IC, Ling ET, Prentice RS | Burkitt’s lymphoma of the appendix: report of two cases | 1980 | Hum Pathol | USA |
| Authors . | Title . | Year . | Journal . | Country . | |
|---|---|---|---|---|---|
| 1 | Yahya HA, Kumar V, Lam JT | Burkitt Lymphoma of the Appendix Presenting With Acute Appendicitis and Acute Kidney Injury: A Case Report and Review of Literature | 2022 | Cureus | USA |
| 2 | Shaw T, Cockrell H, Panchal R, Abraham A, Sawaya D | Burkitt Lymphoma Presenting as Perforated Appendicitis | 2022 | Am Surg | USA |
| 3 | Shahmanyan D, Saway B, Palmerton H, Rudderow JS, Reed CM, Wattsman TA, Faulks ER, Collier BR, Budin RE, Hamill ME | Burkitt-type lymphoma incidentally found as the cause of acute appendicitis: a case report and review of literature | 2021 | Surg Case Rep | USA |
| 4 | Dashottar S, Sunita BS, Singh RK, Rana V, Suhag V, Singh AK | A case series of unusual presentations of Burkitt’s lymphoma | 2020 | J Cancer Res Ther | India |
| 5 | Mimery AH, Jabbour J, Sykes B, MacDermid E, Al-Askari M, De Clercq S | Burkitt Leukemia Presenting as Acute Appendicitis: A Case Report and Literature Review | 2020 | Am J Case Rep | USA |
| 6 | Nam S, Kang J, Choi SE, Kim YR, Baik SH, Sohn SK | Xanthogranulomatous Appendicitis Mimicking Residual Burkitt’s Lymphoma After Chemotherapy | 2016 | Ann Coloproctol | South Korea |
| 7 | Sangma MM, Dasiah SD, Ashok AJ | Ileo-Colic Burkitt Lymphoma in a Young Adult Female- A Case Report | 2016 | J Clin Diagn Res | India |
| 8 | Vrancx S, Van de Sande J, Vanclooster P, de Gheldere C, Van de Velde A | Burkitt Lymphoma Mimicking an Acute Appendicitis in a 17 Years Old Boy: a Case Report | 2015 | Acta Chir Belg | Belgium |
| 9 | Weledji EP, Ngowe MN, Abba JS | Burkitt’s lymphoma masquerading as appendicitis—two case reports and review of the literature | 2014 | World J Surg Oncol | Cameroon |
| 10 | Bhardwaj N, Bains SK, Ortonowski G, Murphy P | A case of Burkitt’s lymphoma presenting as suspected acute appendicitis | 2010 | Afr J Paediatr Surg | India |
| 11 | Wang SM, Huang FC, Wu CH, Ko SF, Lee SY, Hsiao CC | Ileocecal Burkitt’s lymphoma presenting as ileocolic intussusception with appendiceal invagination and acute appendicitis | 2010 | J Formos Med Assoc | Taiwan |
| 13 | Caine YG, Peylan-Ramu N, Livoff AF, Schiller M | Primary Burkitt’s lymphoma of the appendix | 1990 | Z Kinderchir | Germany |
| 14 | Ghani SA, Syed N, Tan PE | A rare cause of acute appendicitis: Burkitt’s lymphoma of the appendix | 1984 | Med J Malaysia | Malaysia |
| 15 | Nanji AA, Anderson FH | Burkitt’s lymphoma with acute appendicitis | 1983 | Arch Surg | Canada |
| 16 | Sin IC, Ling ET, Prentice RS | Burkitt’s lymphoma of the appendix: report of two cases | 1980 | Hum Pathol | USA |
The search criteria (‘Burkitt lymphoma’ [All Fields]) AND (‘appendicitis’ [All Fields]) were applied in databases such as PubMed, Scopus, and Google Scholar. Articles that were not case reports or case series were excluded, such as those without full-text availability in English or Spanish or those not indexed in the databases above.
Conclusions
This case highlights a key surgical oncology dilemma: should gastrointestinal lymphomas be operated on when discovered incidentally? Although chemotherapy is the mainstay of BL treatment, surgery resolved the acute abdomen and enabled diagnosis. The appendectomy was diagnostic—not curative—but facilitated early oncologic care. In the era of nonoperative appendicitis management, surgeons must remain alert to unexpected malignancies with major prognostic implications.
Conflict of interest statement
None declared.
Funding
None declared.



