-
PDF
- Split View
-
Views
-
Cite
Cite
Marah Mansour, Ola Souliman, Sawsan Ismail, Tamim Alsuliman, Fadi Souleiman, A gastric duplication cyst incidentally discovered during an obesity surgery for a 55-year-old female: a rare case report from Syria, Journal of Surgical Case Reports, Volume 2020, Issue 7, July 2020, rjaa215, https://doi.org/10.1093/jscr/rjaa215
Close - Share Icon Share
Abstract
A gastric duplication cyst (GDC) is an uncommon gastrointestinal (GI) tract anomaly in adults. Nonspecific symptoms make it difficult to diagnose, while radiological examination might be helpful. However, the final diagnosis is made after eradication and histopathological study. Herein we report a case of an asymptomatic, incidentally diagnosed gastric duplication cyst discovered during laparoscopic sleeve gastrectomy in a 55-year-old female.
INTRODUCTION
Gastric duplication cyst (GDC) is a very rare gastrointestinal (GI) tract anomaly that represents 4% of all GI tract duplications. Most of the duplications and 67% of cysts of the GI will be diagnosed during the first year of life [1, 2]. It is diagnosed mainly in the ileum, but it can be diagnosed in any part of the GI tract. It can compress the adjacent and nearby organs and may cause symptoms like gastric pain or discomfort, nausea and/or vomiting, gastric regurgitations, weight loss, anemia and dyspepsia [3]. Most GDCs have unclear origins. The majority of cases in adults are discovered accidentally through radiological or during a non-related surgery. Moreover, computerized tomography (CT) scan and endoscopic ultrasound (US) can demonstrate a thickening of the gastric wall. A precise diagnosis is difficult to obtain in pre-resection, while a differential diagnosis may vary widely, including gastrointestinal stromal tumors (GIST), endocrine tumors or migratory pancreatic tissues, whereas malignant transformation is rare. GDCs can be tubular or cystic and their complications include infection, bleeding, perforation, fistulation and malignancy [3, 4]. Surgery may be considered the treatment of choice for GDCs.
CASE REPORT
A 55-year-old female suffering from arthralgia and overweight, with no GI complaint, was admitted to the department of general surgery and prepared for laparoscopic sleeve gastrectomy (LSG). Apart from the abovementioned medical history, clinical examination demonstrated no specific anomaly, while laboratory values were within normal limits before the intervention and upper endoscopy were completely normal. During the programmed LSG, a strange tissue formation with clear boundaries and regular edges, measuring ~3 × 2 cm, was discovered. The tissue was situated on the anterior face relatively close to the lesser curvature of the stomach, following the gastroesophageal junction (Fig. 1), with an enlargement in some adjacent lymph nodes. The mass was intraoperatively noncompressible without changing its size or shape in response to various maneuvers which nearly excluded the possibility of being a diverticulum. The formation was also fully adhered to the front wall and the small curvature of the stomach. The patient underwent surgery for mass removal and the LSG was canceled (Fig. 2). Mass complete eradication was accomplished while adjacent lymph nodes were also removed to permit a full histopathological examination. This procedure was important in the light of retained differential diagnosis which may include malignancies and the impossibility, at the moment, to perform an intraoperative histopathological examination. Macroscopic examination revealed an irregular cyst measuring 3 cm in diameter. The histological examination showed a mucosa lined by gastric foveolar epithelium surrounded by a smooth muscle layer, with a respiratory ciliated pseudostratified epithelium content (Fig. 3). The final diagnosis was a GDC isolated from the gastric cavity with no cellular atypia.
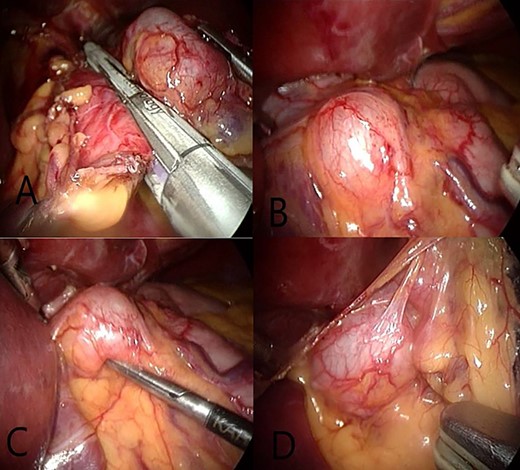
(A and B) A view showing GDCs, a regular shape with clear edges positioned on the front surface and the small curvature of the stomach. (C) We note the maintainance of the shape and size after its compression and try to get it with surgical instruments. (D) After open the small omentum where the posterior surface is observed to GDCs with a number of adjacent lymph nodes.
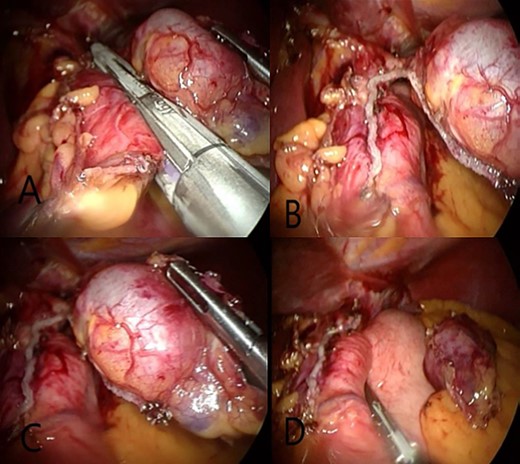
(A) Eradicate GDCs by laparoscopic surgery using endo-GIA special for these cases. (B and C) Separate the GDCs from the stomach while keeping a part connected to the stomach. (D) Complete LSG.
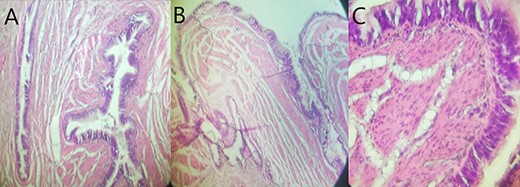
Histological overview using hematoxylin and eosin (H&E) staining demonstrated the cystic wall lined with gastric foveolar epithelium surrounded by a smooth muscle layer with a pseudostratified ciliated epithelial content without cytologic atypia. (A, H&E X 100; B, H&E X 200; C, H&E X 400).
DISCUSSION
With <150 reported cases, GDC represents a very rare congenital malformation [1]. Mostly, the cyst is discovered on the greater curvature of the stomach [5]. In our case, the cyst was located on the lesser curvature and anterior wall of the stomach which is a rarer position. Histologically, gastric foveolar epithelium with multiple glands is usually detected. Pancreatic tissue can also be noticed in this type of cysts [6]. The respiratory pseudostratified epithelium is normally found in esophageal cysts, but it is extremely rare in gastric cysts [3]. In our case, the gastric cyst has gastric mucosa in addition to respiratory epithelium (Fig. 3). There have been about <20 previous cases of GDCs that have respiratory epithelium, 8 of them have both gastric mucosa and respiratory epithelium [3]. In 67% of cases, GDCs are diagnosed during the first year of life and in 95% of them during the first 12 years of life [6, 7]. In our case, we have a 55-year-old completely asymptomatic female which represents a more atypical age for this case diagnosis. Diagnosing GDCs is usually made by a CT scan or US during a routine check. Upper gastric endoscopy can show the cyst impression on the gastric wall. Here, in our case, the endoscopy was completely normal because of the cyst size 3 × 3 cm, and there was no need for a CT scan or US. All blood tests were normal. Differential diagnoses of this cyst included GIST, diverticulum and tumors. During surgery, some maneuvers were made with surgical tools, as aforementioned, to narrow down the possibilities. As final management, the cyst was completely eradicated with the enlarged lymph nodes using laparoscopic surgery. The pathological study demonstrated that the enlarged lymph nodes were reactive. There are three criteria to define gastric duplication: the cyst wall must be contiguous with the stomach wall; the cyst must be girded by a smooth muscle which is contiguous with that of the stomach, and the cyst must be lined by gastric mucosa or any intestinal epithelium [6]. All of these criteria were fulfilled in our case. The final diagnosis is made after complete resection and histopathological examination. In reported cases of GDCs, there were GDCs on the greater curvature with respiratory epithelium [3], GDCs on the lesser curvature with respiratory epithelium and no gastric mucosa [5] or GDCs with some symptoms like epigastric discomfort [8]. GDCs with many complications such as ulceration or malignancy were also reported [4, 9, 10]. In our case a GDC was diagnosed, in an adult female, on the lesser curvature that has both gastric mucosa and respiratory epithelium, completely asymptomatic, discovered during surgery, which makes it, to the best of our knowledge, a unique case in this subject.
CONCLUSION
GDCs are very rare anomalies, where atypical localizations and cellular aspects may be present. Radiological investigations can raise doubts while the proven diagnosis is made after complete eradication and histopathological examination.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflicts of interest.
FUNDING
No funding was required.
ETHICAL APPROVAL
Not required for this case report.
CONSENT
Written informed consent was obtained from the patient for publishing this case report and any accompanying images. A copy of the written consent is available for review by the editor-in-chief of this journal on request.
GUARANTOR
Fadi Souleiman is the guarantor of this work.
AUTHOR’S CONTRIBUTION
M.M.: design of study, data collection, data interpretation and analysis, drafting, critical revision and approval of final manuscript.
O.S.: data collection, data interpretation and analysis, critical revision, drafting and approval of the final manuscript.
S.I.: drafting, critical revision and approval of the final manuscript.
T.A.: the supervisor, drafting, critical revision and approval of the final manuscript.
F.S.: the supervisor; patient care, drafting, critical revision, and approval of the final manuscript.
ACKNOWLEDGEMENT
We are grateful to Mohammad Aloulou for his help in proofreading the manuscript.



