-
PDF
- Split View
-
Views
-
Cite
Cite
A G Kriger, D S Gorin, A R Kaldarov, S V Berelavichus, L A Marinova, G V Galkin, Combination of laparoscopy and endoscopy as an option for treatment patients with gastric neuroendocrine tumors, Journal of Surgical Case Reports, Volume 2019, Issue 1, January 2019, rjz007, https://doi.org/10.1093/jscr/rjz007
Close - Share Icon Share
Abstract
Neuroendocrine tumors (NETs) are relatively rare neoplasms with the increasing survival due to the development of early diagnostics. There is no universal position in treatment and follow up of small (~20 mm) gastric NETs.
Two female patients 51 and 66 y.o. with multiple gastric NETs <2 cm were observed in our department. In both cases treatment was performed by combination of two minimally invasive technologies: laparoscopy and gastroscopy. According to the localization of tumors in one case intraluminal gastric resection controlled by laparoscopy was performed. In the second case laparoscopic gastric resection with gastroscopy assistance was done.
There are two positions for surgical treatment of small NETs: to operate as the typical premalignant neoplasm or to make submucosa resections. We demonstrated combination of laparoscopy and gastroscopy as feasible approach with minimal risk of complications.
INTRODUCTION
Neuroendocrine tumor (NET) is a rare tumor of gut and composes in 5.25 cases for 100 000 populations per year. NETs occur in the 0.1–0.6% of all gastric tumors [1]. In spite of a wide numbers of studies, there is still no universal position in treatment of small (<20 mm) gastric NETs. Some surgeons prefer to follow up without surgery, another ones consider surgical treatment [2, 3]. Combination of laparoscopy and gastroscopy can be an optional approach in treatment of patients with multiple gastric NETs. It helps to avoid large gastric resections an controls the wholeness of gastric wall from abdominal cavity [4].
CLINICAL CASES
Patient G., female 51 y.o., admitted on 22 January 2018. She had not any complaints. The gastric tumor was detected earlier during gastroscopy while the prophylactic medical examination. The diameter of tumor was 16–17 mm.
Histological examination showed G1 gastric NET. The contrast CT revealed two tumors maximal diameter 17 mm (Fig. 1А). In admission patient was in satisfactory condition. On 23 January 2018 the gastroscopy was performed. Seven gastric tumors were found: No. 1, No. 2: 6–10 mm diameter in the upper stomach, No. 3: 5 mm diameter in the gastric body, No. 4, No. 5: 3 mm in the border of anterior wall and major curvature, No. 6: 3 mm diameter in the lower stomach body, No. 7: 17 mm on the border of the antrum and the gastric body between major curvature and anterior wall. All tumors except the major (seventh tumor) were removed during the gastroscopy. Histologycally they were G1 (Ki67 < 0.5%) gastric NETs. Considering the dimensions of the seventh tumor and the possibility of growth among the all layers it was too dangerous to perforate the wall during endoscopy. The decision was to perform combined laparoscopic endoscopic full-flap surgery.
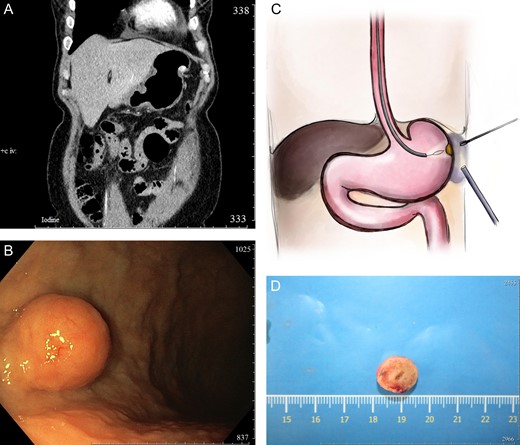
(А) Contrasted abdominal CT, frontal scan, arterial phase. The narrow shows the NET growth all layers of the stomach; (B) intraoperative endoscopic picture; (С) scheme of the procedure; and (D) specimen.
Intraoperative: laparoscopy was performed. The stomach without any pathological findings. All major curvature was mobilized. The submucosa resection scars were visualized which was made before—without any significant. At the same time the gastroscopy started. On the border middle and lower major curvature rounded tumor visualized with the 17 mm diameter. The intraoperative decision was to perform minimally atypical full-flap gastric resection from the mucosa with the laparoscopic navigation and control of the gastric wall. Injections of the indigocarmine were performed around the tumor. The future zone of resection was marked, water preparation and infiltration of the gastric wall layers was made. Using the electro loop minimal atypical gastric resection was performed. The perforation hole with the diameter of 12 mm appeared. Defect was opening into the omentum, was closed using the circular clips. Control from the mucosa—sealed, control from the abdominal cavity—sealed (Fig. 1B and C).
Histology: high differentiated gastric NET arose among the all layers of the stomach wall G1 (Ki67 = 1,5%) (Fig. 1D). Postoperative period was uneventful. Patient was discharged on the third postoperative day. Gastroscopy control after 6 months did not detect any recurrence or significant findings.
Patient Z., 66 y.o. female admitted at 10 May 2017. There were no complaints. During the gastroscopy solid tumor of the antrum on the major curvature with diameter 18 mm. Histological exam showed that it was the high differentiated NET G1 (Ki67 = 1%) of the stomach.
Considering the tumor location, preoperative CT and endosonography data about the tumor’s growing among all layers of the stomach wall we decided to perform laparoscopic atypical gastric resection with the intraoperative endoscopic navigation from the mucosa.
Intraoperative: gastroscopy was performed; the rounded tumor of the antrum visualized with the dimension 18–20 mm. Although there were numerous polyps of the stomach. Polyps were removed endoscopically. The intraoperative navigation was performed. Controlled by the intraoperative gastroscopy and using the gastro lifting the full-flap minimal resection was performed laparoscopic (Fig. 2).
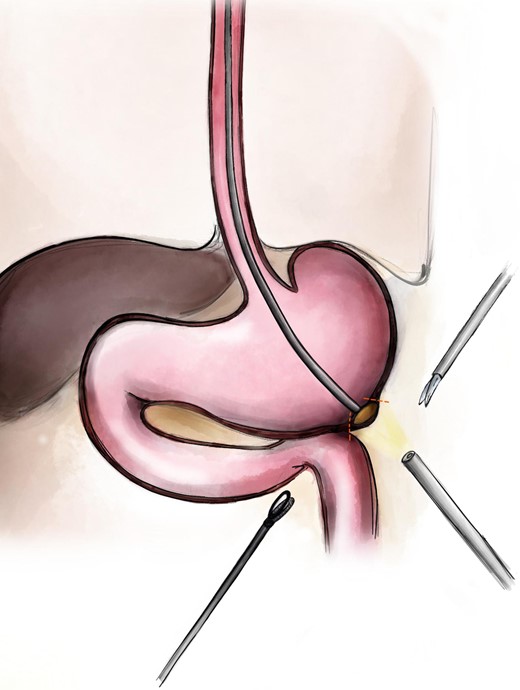
Scheme of the procedure: the endoscope makes the laparolifting to mark the borders of the future resection.
Histological exam with the IHCh confirmed the high grade gastric NET with the Ki67 1%. Postoperative recovery was uneventful. Patient was discharged on the third postoperative day. During the outpatient follow up routine gastroscopy after 3, 6 and 9 months showed no tumor recurrence.
DISCUSSION
For the last years endoscopic technologies provided more in treatment patients with the small benign tumors of the stomach and bowel such as the gastrointestinal stromal tumor or polyps. The small gastric NETs did not become an exception [5]. Nevertheless, considering undefined malignancy potential and the necessity to perform full-fill resection flexible endoscopy has a range of limitations [2].
On another hand, laparoscopy although have more development [6, 7]. The volume of removed gastric tissue can be unreasonably enlarged during laparoscopic resections in cases of small gastric NETs. Combination of the gastroscopy and laparoscopy can become an option of choice when each of techniques shows its best aspects [4].
We demonstrate two cases of combining these techniques. In the first case there was a dangerous of inadequate resection hole closure, the help of laparoscopy was quite significant in the navigation and control after the gastroscopic manipulations. In the second case there was the inverse situation endoscopic navigation and gastro lifting played an important role in fast visualization of the tumor and resection with the minimal trauma. Changing of the light volume became very useful. The flashing from the mucosa allowed to better tumor visualization and decreased the operative time.
As the short term result shows the combined technique provides minimizing time of surgery saves maximal disease free tissue and make the postoperative length of stay minimal and comfortable.
Unfortunately we still have not enough cases to estimate the long term survival. The world literature data are insufficient. In 2014, Y. Yan et al. published the same experience in cases of treatment huge gastric GIST [8]. They although showed feasibility of that combo in treatment patients with benign tumors without depending of the tumor dimension.
The combination of laparoscopy and gastroscopy can become an option for organ preserving surgical treatment not only for small gastric NETs but although for benign tumors of the stomach. Herewith, the dimension of the tumor can be the optional indication for using. Definitely, long-term survival has to be estimated and the comparative trials have to be performed in the future investigations.
CONFLICT OF INTEREST STATEMENT
Authors declare of no conflicts of interest. The authors alone are responsible for the content and writing of the article.



