-
PDF
- Split View
-
Views
-
Cite
Cite
Ming-Jim Yang, David Hernandez-Gonzalo, Elizabeth M Thomas, A case of extensive hepatic adenomatosis in a renal transplant patient, Journal of Surgical Case Reports, Volume 2018, Issue 8, August 2018, rjy195, https://doi.org/10.1093/jscr/rjy195
Close - Share Icon Share
Abstract
Hepatic adenomatosis (HA) is a rare condition that is traditionally associated with oral contraceptive use, glycogen storage diseases or metabolic syndrome. Here we present a renal transplant recipient that was diagnosed with HA and has none of the traditional risk factors. We review the literature on diagnosing and managing HA.
INTRODUCTION
Hepatocellular adenomas (HCA) are primary epithelial neoplasms of the liver that are mostly benign and have been traditionally associated with oral contraceptive (OCP) or steroid use, and more recently, obesity and metabolic syndrome. Hepatic adenomatosis (HA) is a rare condition in which >10 adenomas are found throughout the liver. Here, we present a renal transplant recipient with no traditional risk factors, who presents with an incidental finding of HA.
CASE PRESENTATION
The patient is a 41-year-old female with a past medical history of a living-unrelated renal transplant for end-stage renal disease due to focal glomerulosclerosis. During evaluation for pyelonephritis, a CT scan found multiple non- cystic liver lesions in both lobes. Her medications were significant for maintenance immunosuppression, consisting of tacrolimus 1 mg, cellcept 500 mg, prednisone 5 mg. She denied any history of hepatitis, OCP use or diabetes. Her BMI was 26. She was referred to the hepatobiliary clinic where her initial work-up consisted of lab work, colonoscopy, ultrasound and MRI. Her AFP and CEA were normal at 2.3 and 0.8, respectively. Her colonoscopy was normal. An ultrasound revealed multiple echogenic round lesions in both lobes with no ductal dilatation. An MRI showed ~50 hyper-intense lesions measuring up to 1 cm in both right and left lobes (Fig. 1). A percutaneous liver biopsy was found to be normal hepatic parenchyma. She was managed expectantly for a year until a follow-up MRI showed an increase in size and number of her liver lesions, some of which contained a fatty component.
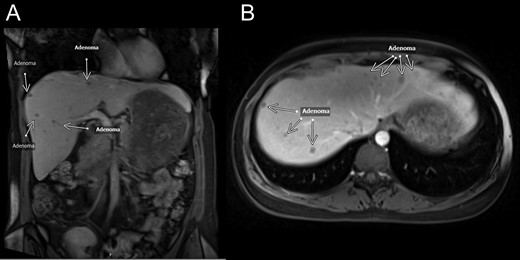
MRI—coronal (A) and transverse (B) MRI sections showing multiple lesions within both lobes of the liver. White arrows mark lesion locations. Per radiology notes, each lesion measured from 0.5 to 1.5 cm in diameter. There were at least 50 lesions in total.
Given the sheer number of her lesions, a multidisciplinary group at our institution decided that a tissue type and specifically a genetic type diagnosis was required to rule out her risk of malignant transformation. A laparoscopic left lateral wedge resection was thus performed in order to obtain adequate tissue for diagnosis. The patient tolerated the procedure well and was discharged from the hospital after a 3-day hospital stay.
Pathological analysis showed well circumscribed, but non-encapsulated liver lesions, comprised of cords of benign hepatocytes (Fig. 2a). The hepatocytes had regular, uniform nuclei and a low nuclear to cystoplasmic ratio. The lesions lacked portal structures and had evidence of unpaired arteries. Moderate microvesicular steatosis was noted within the lesions but none within the uninvolved liver parenchyma. Mitoses or pseudoacinar changes were not identified (Fig. 2b). Immunohistological analysis showed no liver fatty acid binding protein (LFABP) staining (Fig. 2c) and had no nuclear localization of β-catenin (Fig. 2d). These characteristics were consistent with hepatocellular adenoma, HNF1A type.
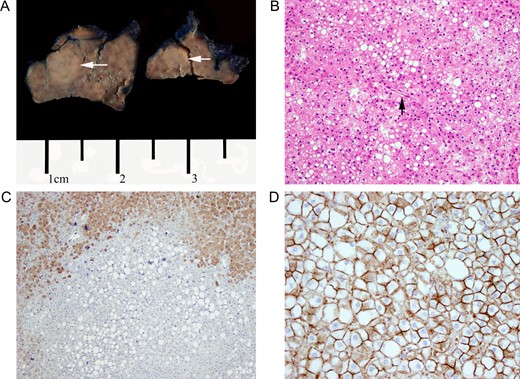
(A) Gross section of left lateral segment showing hepatic adenoma. White arrows indicate adenoma location. (B) H&E stain of hepatic adenoma section. Note diffuse lipid deposition within the adenoma itself and an unpaired artery without other portal structures. Magnification at ×20. (C) LFABP stain. Diffuse LFABP staining was noted within the surrounding liver parenchyma. No staining was seen within the adenoma itself. Magnification at ×10. (D) Beta-catenin staining. Immunochemical stain for beta-catenin showed no nuclear localization. Magnification at ×40.
DISCUSSION
Here, we present a unique case of HA in a renal transplant recipient without any other risk factors. Only one other case of HA in a renal transplant recipient has been described in the literature. Liao et al. described a young man with idiopathic end-stage renal disease who underwent a cadaveric renal transplant. A little more than 3 years after his transplantation, he was found to have multiple lesions in his liver during a routine ultrasound. Just as with our patient, he had no history of hepatitis, diabetes or androgenic steroid use. Per the case report, because there was a concern for possible ‘dysplasia’ on a biopsy, he underwent resection of his tumors. His pathology showed a non-mutated, non-inflammatory hepatocellular adenoma [1].
HA was first described by Flejou et al. in 1985. In their case series, they described five patients who presented with ‘numerous benign adenomas in an otherwise normal hepatic parenchyma’ [2]. At the time, they believed that HA was a distinct entity from singular HCA because it seemed to occur in both men and women, and was less strongly associated with OCPs [2]. Once we were able to molecularly classify the individual tumors, the way we approach and think of HA has changed. Four common genetic subtypes are commonly accepted: (i) HNF1α mutations, (ii) β-catenin mutations, (iii) no mutations but with inflammatory infiltration and (iv) no mutation and no inflammation. Malignant transformation is known to occur in any subtype but most often in the beta-catenin and inflammatory subtypes.
Management of HA now revolves around the molecular phenotype of the individual tumors [3]. Historically, any HCA >5 cm would be considered for resection due to an increased risk for hemorrhage [4, 5]. More recently, Nault et al. has proposed a more personalized approach towards treating HCA’s. Because of the new classification scheme, they proposed treating HCA’s based on their genotype. They recommended surgical resection for high risk lesions such as β-catenin—type HCA’s and/or any HCA >5 cm. Other studies have suggested routinely resecting all male patients with HA [6]. The other types of HCA’s (classes 1, 3, 4) can be managed conservatively with MRI surveillance and by stopping causative agents such as OCPs or androgenic steroids [6]. Because our patient did not have any of the known risk factors or prognostic indicators of HA but her subtype turned out to be an HNF1a subtype, we opted for conservative monitoring with MRI’s annually.
We have thus presented a unique situation in which a patient with a renal transplant on immunosuppression and who has no other traditional risk factors presents with HA. Her case along with the one other case in the literature indicates a possible role that immunosuppression may play in contributing towards adenoma formation. This event is most likely very rare as there are only two case studies at this time. The fact that this occurs though may guide us in understanding the molecular pathogenesis of HCA’s and further elucidate this interesting clinical process.
CONFLICT OF INTEREST STATEMENT
None declared.



