-
PDF
- Split View
-
Views
-
Cite
Cite
Tateki Kubo, Shien Seike, Koichiro Kiya, Ko Hosokawa, Cutaneous resurfacing around a permanent tracheostoma with an internal mammary artery perforator flap, Journal of Surgical Case Reports, Volume 2018, Issue 8, August 2018, rjy183, https://doi.org/10.1093/jscr/rjy183
Close - Share Icon Share
Abstract
When soft tissue reconstruction near a permanent tracheostoma is needed, transfer of a thin and pliable flap is preferable in order to avoid occlusion of the newly created tracheostomal opening. Although microsurgical fasciocutaneous flap transfer may be desirable for such reconstruction, it is not always an option due to lack of recipient vessels for vascular anastomosis or a patient’s poor medical condition that would prohibit a lengthy procedure. An alternative option is the internal mammary artery perforator flap, which is easy to elevate, has a long arc of rotation, and has a reliable blood supply. Here, we report three cases of cutaneous resurfacing around a permanent tracheostoma with an internal mammary artery perforator flap.
INTRODUCTION
Neck soft tissue reconstruction is occasionally required when laryngeal and hypopharyngeal tumor invades surrounding soft tissue or when the skin of the neck is damaged due to previous neck surgery, irradiation and infections secondary to pharyngocutaneous fistulas. When such soft tissue defects are located near the permanent tracheostoma, flap transfer is required. However, such reconstructions are complex and challenging, and bulky flaps would occlude the tracheostomal opening, rendering decannulation impossible (Fig. 1). Thus, transfer of a thin and pliable flap is needed. Here, we report three cases of cutaneous resurfacing around a permanent tracheostoma with an internal mammary artery perforator (IMAP) flap.
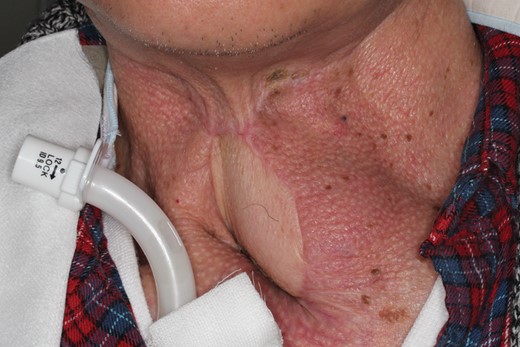
Unfavorable result following resurfacing around the tracheostoma. Cutaneous resurfacing around the tracheostoma was performed with a pectoralis major musculocutaneous flap to repair a pharyngocutaneous fistula. Since the bulky flap obstructs the tracheostomal opening, decannulation is impossible.
CASE REPORT
Case 1
A 76-year-old man receives a tracheoesophageal puncture to recover speech function. However, the postoperative course was unsatisfactory, and the patient suffered repeated infections around the tracheostoma due to an artificially created tracheoesophageal fistula. Therefore, he desired to close the fistula. Shortening of the tracheal stump and new construction of a tracheostoma was planned with cutaneous resurfacing by a 6 × 13 cm IMAP flap based on the second perforator (Fig. 2a and b). A 1/2 circumferential cutaneous portion surrounding the tracheostoma was reconstructed with the IMAP flap (Fig. 2c). The donor site was closed primarily. His postoperative course was uneventful (Fig. 2d).
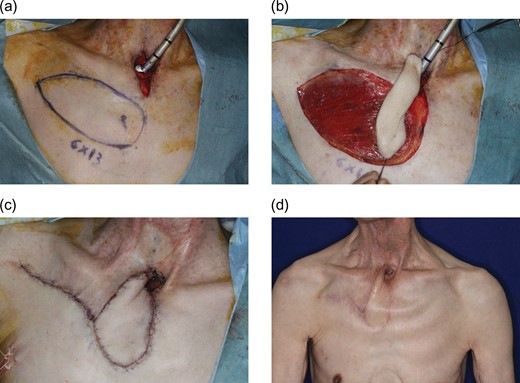
Pre-, intra- and postoperative photographs of Case 1. A 76-year-old man with repeated infections around the tracheostoma due to tracheoesophageal fistula following a failed tracheoesophageal puncture attempt. (a) New tracheostomy was planned with cutaneous resurfacing by a 6 × 13 cm IMAP flap based on the second perforator. (b) Elevated flap. (c) A 1/2 circumferential cutaneous portion surrounding the tracheostomy was reconstructed with the flap. (d) Six-month postoperative view.
Case 2
A 25-year-old woman underwent resection of advanced thyroid cancer and tracheostomal creation. Following a surgical site infection, the neck skin surrounding the tracheostoma became necrotic and the carotid artery was eventually exposed. To cover the carotid artery and to reconstruct neck skin surrounding the tracheostoma, a 15.5 × 6 cm IMAP flap was transferred based on the second perforator (Fig. 3). Approximation of the donor site was possible, and the postoperative course was uneventful.
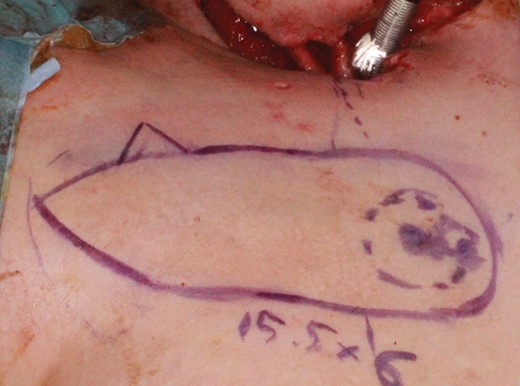
An intraoperative photograph of Case 2. A 25-year-old woman with skin necrosis of the neck and exposure of the carotid artery following surgical site infection. To cover the carotid artery and reconstruct neck skin surrounding the tracheostomy, a 15.5 × 6 cm IMAP flap was designed based on the second perforator.
Case 3
A 72-year-old man with hypopharyngeal cancer underwent pharyngolaryngoesophagectomy. Immediate alimentary tract reconstruction was performed using a free jejunal flap. The neck skin was damaged due to an infection following preoperative tracheostomy. Thus, skin resection was performed. A 16 × 7 cm IMAP flap was transferred with dual pedicles (second and third perforators) (Fig. 4a). A slit was created in the distal flap to accept the tracheostoma circumferentially (Fig. 4b). Skin grafting to the donor site was necessary. His postoperative course was uneventful.
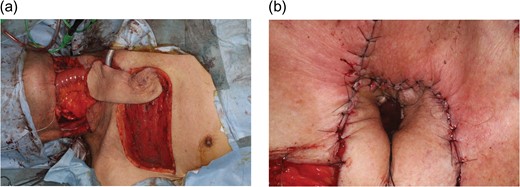
Intraoperative photographs of Case 3. A 72-year-old man with hypopharyngeal cancer underwent pharyngolaryngoesophagectomy, neck skin resection, bilateral neck dissection and free jejunal transfer. (a) A 16 × 7 cm IMAP flap was transferred with dual pedicles (second and third perforators) to reconstruct soft tissue surrounding the tracheostoma. (b) A slit was created in the distal flap to accept a tracheostoma circumferentially without compromising the blood supply.
DISCUSSION
In cases where soft tissue reconstruction near the tracheostoma is necessary, transfer of a thin and pliable flap is preferable in order to avoid occlusion of the tracheostomal opening. The pectoralis major musculocutaneous flap is well-vascularized, is easy to harvest, and easily reaches the neck region. Therefore, the flap is often used for neck reconstruction, especially for the repair of pharyngocutaneous fistulas [1]. However, its volume is inadequate for skin resurfacing near the tracheostoma (Fig. 1). Therefore, transfer of thin and pliable flaps is desirable. Such flaps include free fasciocutaneous flaps such as radial forearm flaps and anterolateral thigh flaps, local skin flaps from adjacent regions and deltopectoral (DP) flaps. In our view, free flaps are the most appropriate, since they can be easily tailored to fit postablative defects and can inset around resected tracheal stumps without difficulty [2]. However, free flap transfer is not always possible due to the lack of recipient vessels for vascular anastomosis. Local skin flaps are unreliable as well, since surrounding skin tissue is often damaged. The DP flap, although rarely used as a first choice, may provide excellent coverage in selected cases [3]. However, in order to provide a reliable blood supply to the flap, as many internal mammary artery perforators as possible are needed at the base of the flap, resulting in an increased width of the flap and decreased arc of rotation. Moreover, skin grafting is usually needed to cover the large area of the anterior chest donor site.
The use of an IMAP flap for anterior neck reconstruction was first described by Yu et al. [3]. Since then, a few articles have been published on this technique [1, 4–9], but IMAP flaps remain a relatively unknown option among head and neck surgeons [1]. This flap is considered to be a refinement of the DP flap [3]. However, one key difference is that the flap can be elevated based on a single perforator as an island flap, whereas the DP flap requires three to four perforators for elevation in order to maintain a reliable blood supply. This feature enables the IMAP flap to have a significantly longer arc of rotation compared to the DP flap. Moreover, direct skin closure is usually possible at the donor site of the IMAP flap, while skin grafting is necessary with the DP flap. Color flow duplex scanning is performed preoperatively to confirm the location and blood flow of the second and third internal mammary perforators. The second perforator is preferable as the basis of the flap because of its proximity to the tracheostoma, as long as the blood flow is not significantly weaker than that of the third perforator [3]. Once the perforator is chosen, the flap is designed obliquely or parallel to the intercostal space [9]. The medial limit of the flap is the midline of the sternum, and the length of the flap is based on the arc of rotation needed to reach the defect with the lateral limit of the flap slightly beyond the anterior axillary fold [3]. If a larger skin area is required, elevation of the IMAP flap with double perforators is possible, as in our Case 3, although skin grafting to the donor site might be needed.
Gilas et al. [10] reported a higher rate of complications in DP flap reconstructions around the tracheostomal site when a slit was created in the distal flap to accept the tracheostoma. McCarthy et al. [2] also advocated framing the tracheal stump with the distal margins of the flap, rather than incising the flap to inset the trachea when using a DP flap for tracheostomal reconstruction. However, in our Case 3, a slit was created in the distal flap to accept the tracheostoma without compromising the blood supply. We speculate that this flap had sufficient blood supply from the dual perforators. In addition, the flap did not require a random vascular supply, which is usually included as the lateral part of the DP flap and is supplied by perforating vessels from the deltoid muscle. Since the IMAP flap has a long arc of rotation, such distal regions are not needed.
In conclusion, the IMAP flap is useful for anterior neck reconstruction, especially for the region surrounding the permanent tracheostoma, where thin and pliable skin is preferable for reconstruction. Therefore, in cases where microsurgical free flap transfer is not available due to the lack of recipient vessels for vascular anastomosis, IMAP flaps are a viable alternative.
CONFLICT OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.



