-
PDF
- Split View
-
Views
-
Cite
Cite
Michail Papoulas, Sawsan Abdul-Hamid, Praveen Peddu, Corina Cotoi, Nigel Heaton, Krishna Menon, Irreversible electroporation in borderline resectable pancreatic adenocarcinoma for margin accentuation, Journal of Surgical Case Reports, Volume 2018, Issue 6, June 2018, rjy127, https://doi.org/10.1093/jscr/rjy127
Close - Share Icon Share
Abstract
Achieving clear microscopic resection margins following pancreaticoduodenectomy (PD) is challenging particularly in borderline resectable pancreatic carcinoma (BRPC). Positive resection margins has been identified as a major independent prognostic factor. Irreversible electroporation (IRE) has emerged as a promising non-thermal ablative method that could be used in the treatment of pancreatic cancer as an adjunct to chemotherapy and surgery. This case report describes the successful simultaneous intraoperative IRE and PD in a patient with BRPC, achieving clear microscopic resection margins. Technical aspects and histology showing the effect of IRE are presented. The role of IRE in the treatment of pancreatic adenocarcinoma should be further evaluated in prospective studies.
INTRODUCTION
Pancreatic ductal adenocarcinoma is the fourth leading cause of tumour-related death in the Western world. Complete tumour extirpation (R0 resection) remains the best possibility for long-term survival. Unfortunately, ~80% of patients are not amenable to resection at presentation either because of metastatic (40%) or locally advanced disease (40%) [1]. In borderline resectable pancreatic cancer (BRPC), local recurrences occur in 11–42% of apparent radical resections [2]. Despite the optimization of the management of patients with BRPC with newer chemotherapeutics and radiation, the prognosis is still dismal [3]. Recent reports of IRE, a novel non-thermal ablative method, have shown the modality to be safe and potentially beneficial to prognosis [4, 5]. We present a case report of successful IRE margin accentuation in a patient with BRPC with particular emphasis on the surgical technique and histology findings.
CASE REPORT
A 61-year-old man diagnosed in January 2016 with a T4N1M0 BRPC in the uncinate process of the pancreas with venous involvement and complete occlusion of the superior mesenteric vein (SMV) and no evidence of metastatic disease (Fig. 1).
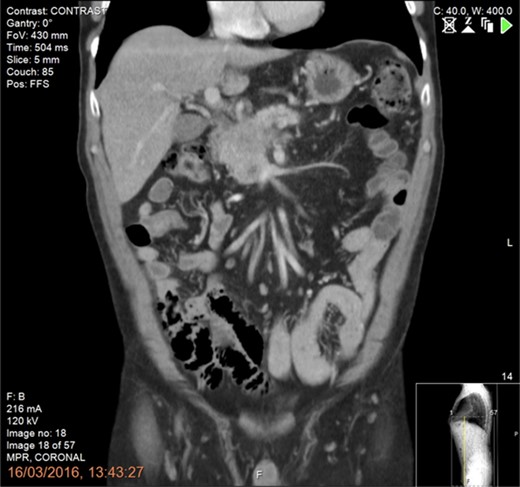
CT scan of the abdomen and pelvis (portal phase) demonstrating a borderline resectable pancreatic tumour at the uncinate process with involvement and complete occlusion of the superior mesenteric vein (SMV) and abutment of the superior mesenteric artery.
Following tissue confirmation, the patient was treated with neoadjuvant chemotherapy including 12 cycles of FOLFIRINOX. Re-staging CT scan showed good response with a recanalized portal vein without any evidence of vascular involvement or distant metastasis (Fig. 2). The patient was then offered a pancreaticoduodenectomy (PD) with simultaneous intraoperative IRE margin accentuation.
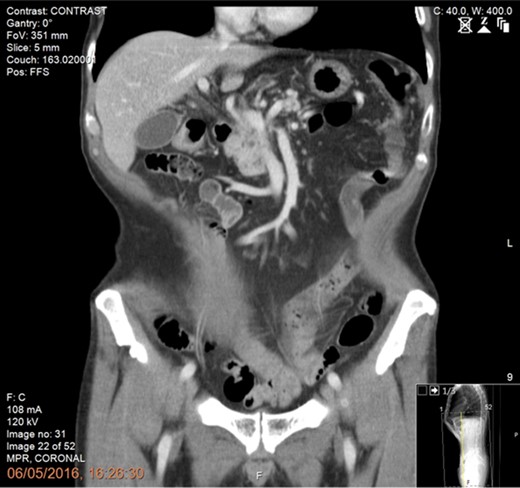
Preoperative CT scan of the abdomen and pelvis (portal phase) showing good response following neoadjuvant chemotherapy with recanalization of the portal vein and no evidence of vascular involvement or distant metastasis.
Procedure
The patient underwent laparotomy, thorough exploration to rule out occult metastases followed by assessment of the tumour and its relation to the SMV/PV confluence and SMA using intraoperative ultrasound. Following kocherization, intraoperative IRE was performed with the guidance and presence of a consultant hepatopancreatobiliary interventional radiologist. We used two probes placed exactly 2 cm apart, at the level of the pancreatic neck under continuous ultrasound guidance. Placement of the needles should be atraumatic, avoiding injuries of underlying vital structures (PV, SMV, SMA). Since the purpose of the IRE was augmentation/accentuation of the resection margins and not in situ IRE of the tumour, we used only one pair of probes.
The probes were initially placed using the anterior and caudal to cephalad approaches along the SMV axis and between the SMV and SMA [6] (Fig. 3a and b). We used a 1.5 cm probe exposure to avoid high current conditions and potential thermal damage. Sequential pullbacks were performed in order to obtain adequate margins both superiorly and inferiorly. Standard default voltage of 1 500 V/cm is initiated with planned delivery of 90 pulses and a pulse width of 70–90 us. The current amperage draw was between 30 and 40 amps, which indicates a safe and effective electroporation. A posterior transduodenal approach was also used in order to accentuate posterior margin (Fig. 3c).
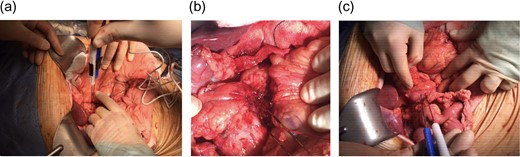
Intraoperative irreversible electroporation (IRE) margin accentuation using one pair of probes. The technique included probe placement using three different approaches including the anteroposterior (a), the caudo-cephalad between the superior mesenteric artery (SMA) and vein (SMV) (b) and the transduodenal (c) in order to augment the pancreatic, the SMV/ SMA groove and the posterior margins.
Following the intraoperative IRE, a classic Whipple’s procedure with portal vein resection and reconstruction using a cadaveric vein was performed.
Histology
Final histology, using the Leeds protocol and the recommendations of the Royal College of Pathologists, revealed a ypT3N0M0 (UICC TNM Seventh Edition) moderately differentiated pancreatic ductal adenocarcinoma [7] (Fig. 4a and b). All resection margins including the posterior margin and the SMA/ SMV bed were negative (Fig. 5a and b). None of the 23 lymph nodes resected was involved by tumour.
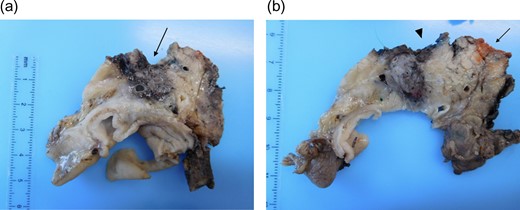
(a and b) Pancreaticoduodenectomy specimen sliced in an axial plane, according to the Leeds Pathology Protocol, providing good visualization of the tumour and its relationship to key anatomical structures and the anterior, posterior and superior mesenteric vein groove surfaces. (a) Demonstrates an area of haemorrhage/necrosis due to IRE needles extending to the SMV bed (arrow) and (b) shows the SMV (arrrowhead) and the SMA groove (arrow).
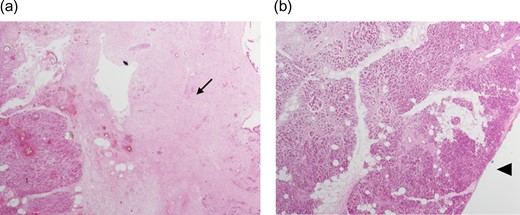
(a and b) Histopathology using haematoxylin and eosin stain showing extensive area of necrosis (arrow) without any viable tumour at the SMV bed (arrowhead).
OUTCOME
There were no major postoperative complication and no IRE related complications. Portal vein graft patency was confirmed postoperatively on imaging and there was no evidence of pancreatitis. Length of hospital stay was 11 days. The patient is currently 12 months post resection without radiological or clinical evidence of recurrent disease.
DISCUSSION
Survival after surgery of pancreatic cancer is still poor, even after curative resection. Positive resection margins, lymph node ratio ≥0.2, and poor differentiation of the tumour have been identified as independent prognostic factors [8]. Published data on the incidence of margin involvement vary widely between studies, from below 20% to over 75% [9]. Menon et al.[7] reported an 85% R1 rate for pancreatic cancer following the use of the rigorous, fully standardized Leeds Pathology Protocol (LEEPP).
Achieving clear microscopic resection margins following PD is challenging particularly in BRPC with mesenteric vasculature involvement [4, 10]. In view of the pressing need for optimal local disease control, irreversible electroporation (IRE) has emerged as a novel, non-thermal ablative therapy of pancreatic cancer [3, 4, 6]. Electrodes are placed around the tumour, and a pulsed, direct current with a field strength of 1500 V/cm is delivered. The application of electric field across a cell alters the transmembrane potential. This results in disruption of the lipid bilayer and creation of small nanopores in cell membrane. Nanopores allow micro- and macromolecules to be transported into and out of the cell. This causes apoptosis and cell death without significant heating of the tissues, which spares the extracellular matrix and proteins. In contrast to the traditional ablative methods, IRE could be used safely near vascular and ductal structures.
The role of IRE in the treatment of pancreatic cancer is versatile [8]. IRE could be considered as an adjunct to chemotherapy, chemoradiotherapy and pancreatectomy. In patients with LAPC the addition of IRE to conventional therapies results in substantially prolonged survival compared to historical controls [3, 4, 6]. Intraoperative IRE margin accentuation and pancreatectomy in BRPC could minimize the risk of local recurrence and improve outcome. Further indications for treatment with IRE are control of local recurrence after previous Whipple procedure, and/or intolerance to systemic chemotherapy and resectable pancreatic cancer in patients not fit for surgery.
The present case report describes the first successful surgical resection (R0 margin) of a borderline resectable pancreatic cancer using IRE for margin accentuation in the UK. The patient remains free of disease without any local or distant metastasis 12 months following PD with IRE.
CONCLUSION
Simultaneous intraoperative IRE and PD could be used successfully in appropriate patients with BRPC in order to achieve clear microscopic resection margins. The role of IRE in the treatment and management of pancreatic adenocarcinoma should be further evaluated in prospective studies.
CONFLICT OF INTEREST STATEMENT
The authors have no conflict of interest.
REFERENCES
Author notes
Michail Papoulas and Sawsan Abdul-Hamid contributed equally to the study.



