-
PDF
- Split View
-
Views
-
Cite
Cite
Alexander Kohler, Thilo Welsch, Anne-Kathrin Sturm, Gustavo B Baretton, Christoph Reissfelder, Jürgen Weitz, Carina Riediger, Primary choriocarcinoma of the liver: a rare, but important differential diagnosis of liver lesions, Journal of Surgical Case Reports, Volume 2018, Issue 4, April 2018, rjy068, https://doi.org/10.1093/jscr/rjy068
Close - Share Icon Share
Abstract
The case of a 64-year-old man with spontaneous acute abdominal bleeding is presented. Under suspicion of an atypical hepatocellular carcinoma an extended left hemihepatectomy was performed. Histological diagnosis after surgical therapy revealed a primary hepatic choriocarcinoma. During follow-up within 5 months several metastases were detected. Because of the number and localization of the metastases, there was no further curative surgical option and palliative systemic chemotherapy was initiated. Primary choriocarcinoma of the liver is an important differential-diagnosis in hypervascularized lesions of the liver. This tumor-entity is highly aggressive, with rapid tendency for metastatic spreading. This correlates with the poor prognosis of 12 months after tumor-detection.
INTRODUCTION
Choriocarcinoma is a rare germ-cell tumor that can be located gonadal and extragonadal. Gonadal choriocarcinoma represents <0.5% of all gonadal tumors in male patients and is even rarer in female patients. Extragonadal manifestations can occur in liver, retroperitoneum, mediastinum, lung or small bowel. Choriocarcinoma of the liver is characteristically hypervascularized and often leads to spontaneous abdominal bleedings.
We present the case of a 64-year-old man with spontaneous acute abdominal bleeding of primary hepatic choriocarcinoma under anticoagulant therapy who received extended left hemihepatectomy under suspicion of atypical hepatocellular carcinoma.
CASE REPORT
We report the case of a 64-year-old white male with an extragonadal choriocarcinoma of the liver.
The patient was admitted to our hospital due to spontaneous intra-abdominal bleeding from two hypervascularised liver-lesions located in segment IV in March 2016. He was therapeutically anticoagulated for paroxysmal pre-shimmering. Due to abdominal pain an abdominal sonography was performed which showed a subcapsulary bleeding. CT-scan confirmed active bleeding from the central liver lesion. The patient was transferred to our facility. The hemoglobin level of the patient at admission was 4.4 mmol/l.
Patient’s medical history revealed that those hypervascularized hepatic tumors have been known since August 2015. Two biopsies of the tumor taken in an external hospital in August 2015 did not show any sign of malignancy and were histologically proven adenoma. At the time of admission to our hospital in March 2016, the patient was in excellent general condition without any signs of weight loss or general weakness. Laboratory findings showed α-fetoprotein, carcinoembryonic antigen and carbohydrate antigen 19-9 levels within normal range. Human chorionic gonadotropin (hCG) was not analyzed.
The primary differential diagnosis was bleeding episode of liver adenoma—misled by the previously obtained histopathology report. The bleeding ceased after a diagnostical angiography and a conservative treatment with follow-up exams was initiated.
An elective control computed tomography scan (Fig. 1a) in April 2016 revealed constant hypervascularized lesions still not showing signs of hepatocellular carcinoma. Because of a suspected atypical hepatocellular carcinoma, an additional magnetic resonance imaging (MRI) study was performed (Fig. 1b) and a surgical resection was indicated. The intraoperative exploration showed a large central tumor of the liver infiltrating the left lobe and surprisingly the diaphragm. Extended left hemihepatectomy with partial resection of the diaphragm, cholecystectomy and biliary reconstruction by an end-to-side hepaticojejunostomy was performed on 3 May 2016 (see Fig. 4, resection line and tumor-location).
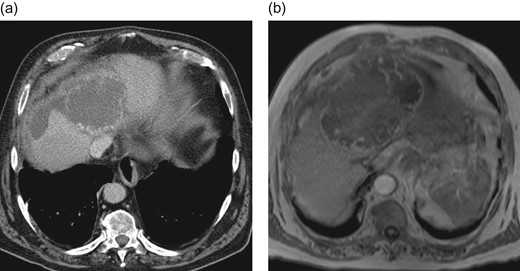
Preoperative CT-scan (a) and MRI (b) of the liver showing hypervascularized hepatic lesions with central necrosis and associated hematoma.
Intraoperatively obtained fresh frozen sections revealed unclear pathological lesions with distinctive necrotic areas. The postoperative clinical course was uncomplicated. The release from hospital was possible 20 days after surgery.
Postoperative histological assessment revealed infiltration of an extragonadal choriocarcinoma (Figs 2 and 3) measuring 14.5 cm in diameter with destructive growth, distinctive cystoid hemorrhagic infarctions. Resection margins were free of tumor-infiltration. Immunohistochemical staining revealed expression of hCG (Fig. 3b), Inhibin-expression especially in syncytiotrophoblastic cells, epithelial membrane antigen (EMA), pan-cytokeratin (Pan-CK), showing epithelial origin and lack of reaction against Ets related gene (ERG), for exclusion of a vascular malformation and Arginase-1, for exclusion of a liver-own tumor, leading to the diagnosis of a choriocarcinoma (Fig. 4).
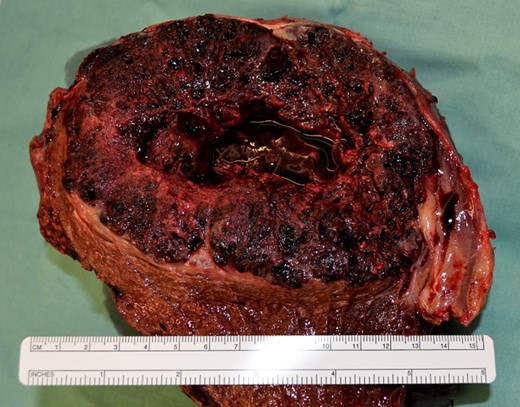
Intraoperative liver specimen showing the large tumor (divided on the back table).
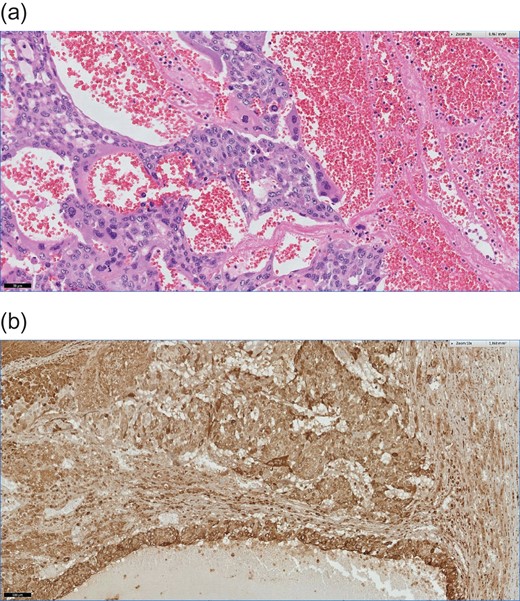
(a) Formaline-embed sections, Hematoxylin eosin (HE) staining, ×200.This photomicrograph shows an extensive hemorrhage and typically features of a choriocarcinoma. The tumor composed of mononucleate cytotrophoblasts and multinucleate syncytiotrophoblasts. (b) Formaline-embed sections, immonhistochemical (IHC) staining using anti-ß-hCG antibodies, ×100. The tumor cells are diffusely positive for ß-hCG.
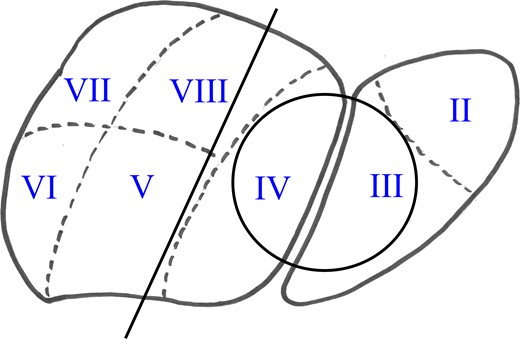
Liver diagram showing lesion (circle) and resection line (Segments I–VI were resected).
Because of the histological results the ß-hCG serum levels and a complete urological staging was performed. Postoperative serum hCG levels were highly elevated >5000 U/ml. There was neither a testicular primary tumor nor any other extrahepatic tumor manifestation.
Additional fludeoxyglucose-positron emission tomography (FDG-PET/CT) to complete the staging examination four weeks postoperatively revealed new and suspicious bipulmonary lesions as well as enlarged iliacal lymph nodes. As secondary diagnosis, pulmonary embolism was seen. This led to a re-admission and subsequent inpatient treatment.
Those findings pointed to an aggressive disease course with early relapse and rapid progression of the disease.
Because of the number and localization of the metastases, there was no further curative surgical option and palliative systemic chemotherapy was initiated. The patient was treated with four cycles of chemotherapy according to the PEI scheme (cisplatine/etoposide/ifosfamide) until August 2016. Afterwards he received additively pegfligrastim.
During the chemotherapy he developed a severe aplasia leading to uncontrollable sepsis with subsequent multiorgan failure. The patient died at 18 September 2016.
DISCUSSION AND MINI-REVIEW
Choriocarcinoma is a rare germ-cell tumor that mostly occurs gonadal. Only few cases of primarily extragonadal choriocarcinomas are reported. Men seem to be affected more often than women.
Extragonadal primary choriocarcinoma can occur in liver, lung, stomach and small bowel. Whether they develop primarily in the retroperitoneum or other organs or whether they are metastases of a primary gonadal tumor has been debated for a long time [1].
Primary choriocarcinoma of the liver is an aggressive malignancy frequently associated with clinical instability at initial presentation [2]. The first description of extragonadal, primarily hepatic choriocarcinoma was published in 1992 [3]. So far 10 cases of primary choriocarcinomas of the liver are published in the literature including a case series of five cases by Shi et al. [4] (Table 1). Regarding literature, cases of one female and nine male patients with primary hepatic choriocarcinoma are reported with a median age of 49.3 years (range 36–65 years). Most common symptoms were abdominal pain, rapid liver growth, cachexia, bleeding as shown in our presented case.
Summary of all cases of primary choriocarcinoma of the liver published in the literature.
| Study . | Year of report . | Patients age . | Survival after diagnosis . |
|---|---|---|---|
| Fernandez Alonso et al. [3] | 1992 | 62 | 12 months |
| Arai et al. [5] | 2001 | 65 | 45 days |
| Shi et al. [4] | 2010 | 39 | 6 months |
| Shi et al. [4] | 2010 | 45 | 2 months |
| Shi et al. [4] | 2010 | 48 | 3 months |
| Shi et al. [4] | 2010 | 36 | 5 months |
| Shi et al. [4] | 2010 | 40 | 8 months |
| Bakhshi et al. [6] | 2012 | 40 | 10 days |
| Sekine et al. [7] | 2013 | 49 | 2 months |
| Malikov et al. [8] | 2015 | 54 Females | Surgery and adjuvant chemotherapy (vincristine and cyclophosphamide) unknown |
| Present case | 2016 | 64 | 5 months |
| Study . | Year of report . | Patients age . | Survival after diagnosis . |
|---|---|---|---|
| Fernandez Alonso et al. [3] | 1992 | 62 | 12 months |
| Arai et al. [5] | 2001 | 65 | 45 days |
| Shi et al. [4] | 2010 | 39 | 6 months |
| Shi et al. [4] | 2010 | 45 | 2 months |
| Shi et al. [4] | 2010 | 48 | 3 months |
| Shi et al. [4] | 2010 | 36 | 5 months |
| Shi et al. [4] | 2010 | 40 | 8 months |
| Bakhshi et al. [6] | 2012 | 40 | 10 days |
| Sekine et al. [7] | 2013 | 49 | 2 months |
| Malikov et al. [8] | 2015 | 54 Females | Surgery and adjuvant chemotherapy (vincristine and cyclophosphamide) unknown |
| Present case | 2016 | 64 | 5 months |
Summary of all cases of primary choriocarcinoma of the liver published in the literature.
| Study . | Year of report . | Patients age . | Survival after diagnosis . |
|---|---|---|---|
| Fernandez Alonso et al. [3] | 1992 | 62 | 12 months |
| Arai et al. [5] | 2001 | 65 | 45 days |
| Shi et al. [4] | 2010 | 39 | 6 months |
| Shi et al. [4] | 2010 | 45 | 2 months |
| Shi et al. [4] | 2010 | 48 | 3 months |
| Shi et al. [4] | 2010 | 36 | 5 months |
| Shi et al. [4] | 2010 | 40 | 8 months |
| Bakhshi et al. [6] | 2012 | 40 | 10 days |
| Sekine et al. [7] | 2013 | 49 | 2 months |
| Malikov et al. [8] | 2015 | 54 Females | Surgery and adjuvant chemotherapy (vincristine and cyclophosphamide) unknown |
| Present case | 2016 | 64 | 5 months |
| Study . | Year of report . | Patients age . | Survival after diagnosis . |
|---|---|---|---|
| Fernandez Alonso et al. [3] | 1992 | 62 | 12 months |
| Arai et al. [5] | 2001 | 65 | 45 days |
| Shi et al. [4] | 2010 | 39 | 6 months |
| Shi et al. [4] | 2010 | 45 | 2 months |
| Shi et al. [4] | 2010 | 48 | 3 months |
| Shi et al. [4] | 2010 | 36 | 5 months |
| Shi et al. [4] | 2010 | 40 | 8 months |
| Bakhshi et al. [6] | 2012 | 40 | 10 days |
| Sekine et al. [7] | 2013 | 49 | 2 months |
| Malikov et al. [8] | 2015 | 54 Females | Surgery and adjuvant chemotherapy (vincristine and cyclophosphamide) unknown |
| Present case | 2016 | 64 | 5 months |
Choriocarcinoma as differential diagnosis of hypervasculariszed lesions, like bleeding adenomas or hepatocellular cancer, is very important. The screening using ß-hCG is a practical and effective approach in diagnosing choriocarcinomas.
Similar to our case, laboratory findings in all reported cases showed excessive elevated hCG levels. In contrast, α-fetoprotein, carcinoembryonic antigen and carbohydrate antigen 19-9 levels were within normal ranges [8]. Tumor markers show a typical pattern in choriocarcinoma: ß-HCG is always elevated, whilst alpha-fetoprotein and alkaline phosphatase are always negative.
In CT scan and MRI choriocarcinomas present as hypervascularized liver lesions. In differential diagnosis liver adenomas, liver hematomas, hepatocellular carcinomas or secondary liver malignancies have to be taken into account. In adults its differential diagnoses can be metastatic liver tumor or intrahepatic cholangiocarcinoma [7]. In children it often mimics other benign and malignant childhood liver tumors [2].
Consequently, screening of serum hCG is an excellent diagnostic tool in case of unclear hypervascularised lesions with or without necrotic areas of the liver. It could be shown that additional FDG/PET/CT in course after treatment is useful for detecting syn- and metachronous lesions. Furthermore, unusual locations of choriocarcinoma can also be detected [9].
Pathological and histolopathological characteristics were similar in all reported cases showing large diameters with soft, hemorrhagic tissue and areas of necrosis. Histologically, tumors consist of solid papillary proliferation of cytotrophoblastical cells, without secretion of hCG, which are coated by syncytiotrophoblastical giant-cells with hCG secretion [10]. Neoplastic cells were positive for human chorionic gonadotropin and negative for alpha-fetoprotein and carcinoembryonic antigen [3–5]. The absence of a tumorspecific stroma leads to early vascular infiltration and invasion with accompanying early seeding of distant metastasis. Therefore, choriocarcinoma is considered highly malignant, with poor prognosis [10]. Our histological findings extragonadal choriocarcinoma do not differ from histological characteristics of gonadal choriocarcinoma that were shown above. An exception in prognosis is seen in choriocarcinoma of the pregnancy, with myometrial growth, because of partial paternal antigenpattern, this choriocarcinoma is treated as transplant-tissue by patients’ immune-system.
Surgery has been proved to be the only effective and enduring therapeutic option. A survey of malignant germ-cell tumors presented that primary site in the gonads were favorable prognostic factors, whereas histologic findings of choriocarcinoma and liver or lung metastasis were unfavorable. Radical complete resection alone is a sufficient treatment [11]. However, surgical options are limited by the extension of resection, and possible complications like choriocarcinoma syndrome, with massive hemorrhage at metastatic sites, which is a rare and life-threatening complication which may occur in patients with primary pulmonary choriocarcinoma or extended metastases.
Adjuvant treatment of choriocarcinoma is similar to other non-seminous germ-cell tumors. Vincristin and cisplatin are favored substances [8].
It can be outlined that this tumor-entity is highly aggressive, with rapid tendency for metastatic spreading. This correlates with the poor prognosis of 12 months after tumor-detection, which is described in literature (Table 1). Further problems result from therapy of extended metastases with chemotherapy, which can lead to tumor-lysis and accompanying mortality. Screening can and should be part of the clinical work-up, using ß-hcG.
In summary, we present the 11th case of primary choriocarcinoma of the liver. The 67-year-old male patient showed initially only slow progression of the disease postulating that adenoma was the underlying disease in 2015 when biopsies were taken. One can discuss whether rapid progression after complete liver resection was induced by surgery or choriocarcinoma developed from an initially benign liver tumor.
CONFLICT OF INTEREST STATEMENT
No conflicts of interest have to be reported.
FUNDING
No conflicts have to be reported.
RESEARCH SUPPORT
All institutes are named, which supported the publication of this case report.
PERMISSIONS
All pictures were obtained by the authors. All authors agree in publishing and using the pictures.
REFERENCES
Author notes
All mentioned authors have contributed to this paper, with surgical, clinical and theoretical contributions.



