-
PDF
- Split View
-
Views
-
Cite
Cite
Emese Irma Ágoston, Áron Somorácz, Lilla Madaras, Attila Zaránd, Gyöngyvér Szentmártoni, Zsuzsanna Orosz, Magdolna Dank, Zsolt Baranyai, Successful treatment of three synchronous primary malignant tumours—reflection on surgical, pathological and oncological aspects and decision making, Journal of Surgical Case Reports, Volume 2018, Issue 4, April 2018, rjy041, https://doi.org/10.1093/jscr/rjy041
Close - Share Icon Share
Abstract
We report a case of a patient with triple synchronous primary malignancies (breast, colon, kidney) which has not been previously reported in the literature. A 70-year-old woman was diagnosed with invasive ductal carcinoma of the left breast with axillary lymph node metastasis. During the staging period, renal cell carcinoma on the left kidney and mucinous adenocarcinoma in the proximal colon were found. Since the breast tumour demonstrated favourable biology, aromatase inhibitor therapy had been started and simultaneous right colectomy and left nephrectomy was performed. Six months after the first diagnosis, left sector excision and axillary block dissection were performed. Adjuvant FEC chemotherapy was administered, followed by radiotherapy. During the 16-month follow-up period disease recurrence was not detected.
INTRODUCTION
The occurrence of multiple primary malignant neoplasms is estimated between 0.734% and 11.7% and shows increasing tendency [1, 2]. According to the Warren and Gates diagnostic criteria, each tumour should present a definite picture of malignancy, they should be histologically distinct and none of these tumours should be the metastasis of the others, diagnosed within 6 months could be defined as synchronous [3, 4]. We report a case of a triple synchronous primary malignancies (breast, colon, kidney) which has not been previously reported in the literature.
CASE REPORT
The present case is of a 70-year-old female patient with positive family history of leukaemia, colon cancer and type 2 diabetes mellitus. In the annual breast cancer screening, the mammography revealed a 1.7 cm × 2.5 cm mass in the upper outer quadrant of her left breast with axillary lymph node metastasis. Core biopsy confirmed invasive breast carcinoma (IBC) with low-grade ductal carcinoma in situ (DCIS), oestrogen and progesterone receptors were both positive (ER 100%, Q-score 7–8, PR 70%, Q-score 3–4), Her2 was negative (Score 0) and Ki67 labelling index was 5%. During the staging period, positron emission tomography-computed tomography (PET-CT) scan was performed and it demonstrated an abnormal FDG uptake in the upper part of the left kidney and in the right colon too (Fig. 1). On colonoscopy, 7–8 polyps were detected and an endophytic tumour in the ascending colon. Biopsy of the latter mass revealed moderately differentiated mucinous adenocarcinoma. Computed tomography confirmed a 3.8 cm × 3.6 cm mass of the left kidney and a 5 cm mass in the right colon extending until the hepatic flexure. (Fig. 2) Percutaneous CT-guided fine needle aspiration of the left kidney showed renal cell carcinoma. The case was referred to the multidisciplinary oncological team.
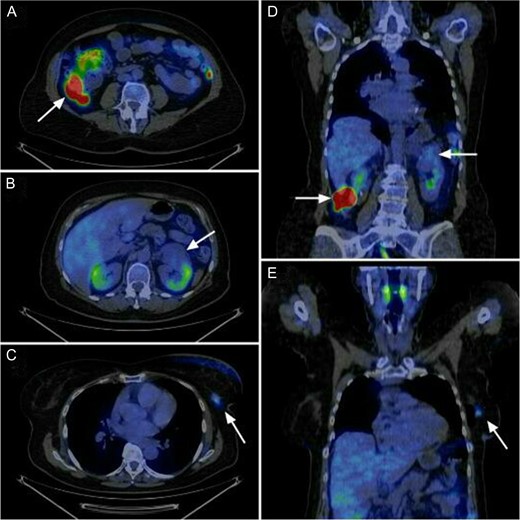
Abnormal FDG uptake in the three localisation according to the sagittal and coronal view of the PET-CT. Axial view shows (A) the colon ascendens tumour, (B) the left kidney tumour and (C) the left breast tumour. Coronal view shows (D) the left kidney tumour and the colon tumour and (E) the left breast tumour.
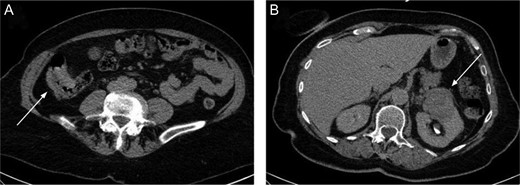
The right colon mass (A) and the left kidney mass (B) on the CT scan.
Since the breast tumour showed favourable biology (Grade 1, invasive ductal carcinoma with hormone receptor positivity and low Ki67 labelling index), our choice was to start her tratment with a simultaneous right hemicolectomy and left nephrectomy. During this period, aromatase inhibitor therapy had been administered for the breast cancer. Six months after the first diagnosis of the breast cancer, left sector excision and axillary block dissection were performed. After an uneventful recovery, six cycle adjuvant 5-fluorouracil, epiadrimicin, cyclophosphamid chemotherapy (FEC scheme) was administered due to lymph node involvement, followed by radiotherapy according to the international protocol. The follow-up period consists of 16 months, the patient is disease free, no tumour recurrence was noted. Postoperative histopathological evaluation of the colon specimen demonstrated a 5 cm, Grade 2, pT3N0 mucinous adenocarcinoma infiltrating the whole thickness of the bowel wall. All 18 lymph nodes were devoid of metastases. Venous invasion was present within the subserosa of the bowel wall. Immunohistochemistry (IHC) revealed loss of expression of MLH1 and PMS2 in tumour cells (but not within stromal cells and lymphocytes), and weak nuclear reaction with MSH6 and MSH2 was observed within tumour cells and internal control cells (stromal cells and lymphocytes). The localisation, the mucinous nature and the histological appearance of the invasive cancer within the ascending colon all pointed toward the involvement of the serrated pathway. For clinical treatment purposes investigation of KRAS and BRAF was advised to define whether this tumour could be resistant to 5FU and anti-EGFR therapies (Fig. 3). By molecular analysis, the tumour showed KRAS mutant and BRAF wild type. The renal specimen comprised a 4.8 cm diameter, ISUP grade 1, pT3a renal cell carcinoma with venous invasion (Fig. 5). The breast specimen revealed a 2.2 cm × 2.0 cm, Grade 2 invasive breast carcinoma (pT2N2a) with low-grade DCIS. Among the nine axillary lymph nodes five demonstrated macrometastases (Fig. 4).
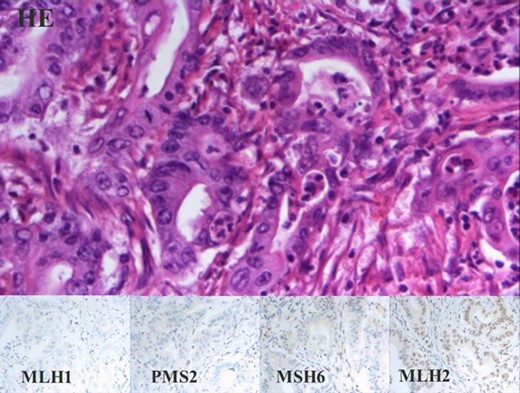
Mucinous adenocarcinoma of the colon. Panel with four columns and two rows. Upper row shows HE staining, lower rows show microsatellite instability with IHC. All of the same 40× magnification.
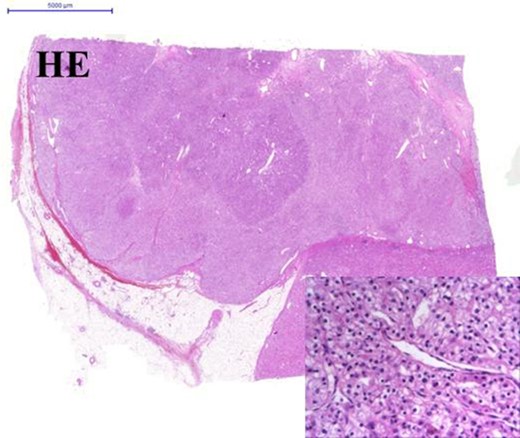
Clear cell carcinoma of the left kidney. Panel with two rows show HE staining. Image in the centre 0.5× and on the lower-right corner 40× magnification.
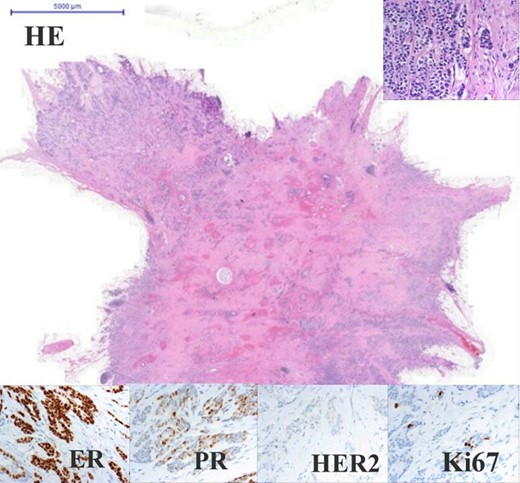
Adenocarcinoma of the left breast. Panel with four columns and three rows. Upper rows show HE staining in the centre 0.5× and on the upper-right corner 40× magnification. Lower rows show IHC all of the same 40× magnification. Oestrogen receptor 100% positive (Q-score 7–8), progesterone receptor 70% positive (Q-score 3–4), Her2 negative (Q-score 0), Ki67 index 5%.
DISCUSSION
Cancer patients have a multiple cancer risk 10% higher in comparison to the general population [5]. Better understanding of the molecular background of tumourgenesis may contribute to establishing other treatment strategies in the future. Previously found that microsatellite unstable cancers have a higher incidence of synchronous and metachronous tumours [6]. In our case colorectal cancer (CRC) showed microsatellite instability, KRAS mutation and BRAF wild type characteristics. Since within the invasive tumour MLH1 and PMS2 protein loss was noticed exclusively within tumour cells, while internal control cells (stromal cells and lymphocytes) had normal protein expression by all 4 IHC, Lynch syndrome could be excluded. We presumed a CpG island metilator phenotype to be the underlying cause. All lesions were locally advanced and following the international guidelines adjuvant treatment of colon and breast cancer were required. Combined adjuvant radio- and chemotherapy (FEC scheme) was given for the breast cancer. Our standpoint was to treat both the colon and breast cancers—as the FEC scheme contains 5FU—with no additional need to other chemotherapeutic agents. Previous reports showed that patients with deficient MMR colon cancers do not benefit from FU-based adjuvant therapy and in stage II CRC it could even be harmful [7, 8]. We find that the given adjuvant therapy was relevant in our case, which was based on the histological, molecular behaviour and pathological stage of each tumour. The therapeutic choice was made possible by the widespread preoperative examination. Although PET-CT scan is not part of the routine preoperative examination, in our case it was eligible according to the National Health Insurance Fund of Hungary. The PET-CT scan provided a complex view on all tumours and the possible metastases which facilitated the appropriate therapeutical decision making. Additionally, a recent study concluded that PET-CT can help in accurate staging and might result in a more specified prognostication compared to the conventional CT imaging [9]. The delay of the surgical treatment of breast cancer was possible due to its favourable biological behaviour and therefore the surgical treatment of kidney and colon tumours could be carried out first. During this period, aromatase inhibitors could be started for the breast cancer based on its receptor positivity. In the aforementioned case, we needed to decide the best strategy of treatment based on the biological behaviour of each tumour. The widespread preoperative examination and the collaboration of the interdisciplinary team played a key role in adopting the potentially most effective and personalised treatment.
CONSENT
Written informed consent was obtained from the patient for publication of this Case report and any accompanying images. A copy of the written consent is available for review by the Editor of this journal.
ACKNOWLEDGEMENTS
The authors thank Péter Szabó for delivering PET-CT images and A. Marcell Szász for digitalisation of the tissue sections. In addition, the authors thank Prof. László Harsányi for his support and the devoted assistance of Erzsébet Rásó and Tamás Barbai for the preparation of slides and her contribution to immunohistochemical reactions.
REFERENCES
- radiation therapy
- aromatase inhibitors
- cancer
- chemotherapy regimen
- adenocarcinoma, mucinous
- immunologic adjuvants
- pharmaceutical adjuvants
- axilla
- breast neoplasms
- infiltrating duct carcinoma
- renal cell carcinoma
- decision making
- tissue dissection
- follow-up
- nephrectomy
- surgical procedures, operative
- breast
- colon
- diagnosis
- kidney
- metastasis, axillary
- colectomy, right
- kidney, left
- excision



