-
PDF
- Split View
-
Views
-
Cite
Cite
Sebahattin Destek, Vahit Onur Gul, Serkan Ahioglu, A variety of gene polymorphisms associated with idiopathic granulomatous mastitis, Journal of Surgical Case Reports, Volume 2016, Issue 9, September 2016, rjw156, https://doi.org/10.1093/jscr/rjw156
Close - Share Icon Share
Abstract
Idiopathic granulomatous mastitis (IGM) is a rare and chronic inflammatory disorder. IGM mimics breast cancer regarding its clinical and radiological features. Etiology of IGM remains unclarified. Our patient was 37-year-old and 14 weeks pregnant. There was pain, redness and swelling in the right breast. The mass suggestive of malignancy was detected in sonography. Serum CA 125 and CA 15-3 levels were high. Genetic analysis was performed for the etiology. methylenetetrahydrofolate reductase (MTHFR) C 677 TT, β-fibrinogen-455 G>A, plasminogen activator inhibitor (PAI)-1 5 G/5 G, angiotensin-converting enzyme (ACE) I/D mutation was found. IGM was diagnosed by cor biopsy. An association was also reported between breast cancer and mutations in MTHFR-C 677 T, PAI-1, ACE genes. Genetic polymorphisms may involve in the development of IGM as it was seen in our case. Further studies should be conducted to better clarify this plausible association.
INTRODUCTION
Idiopathic granulomatous mastitis (IGM) is a rare chronic inflammatory disease of characterized breast occurred by gralunoma, lobulitis and abscess formation [1]. It has an unknown etiology. At present time autoimmune etiology is worked on [1]. Generally, specific microorganism is not determined in abscess culture. There are no radiologic pathognomonic findings. For final diagnosis, histopathological examination is required. Clinical and radiological features are similar to breast carcinomas [2]. Some studies reported that IGM can accompany breast cancer and moreover, long chronic cases can cause breast cancer [2]. In this article, it is emphasized that some genetic disorders which are related to breast cancer may have a role in IGM etiology.
CASE REPORT
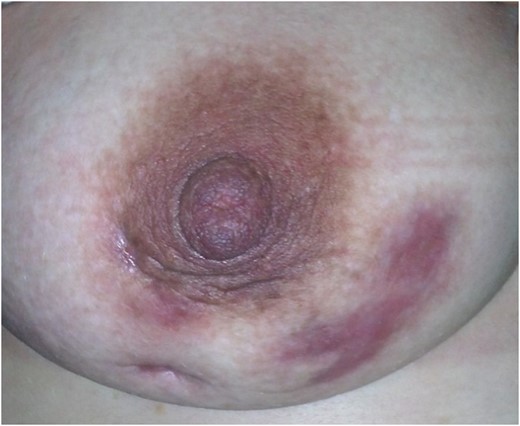
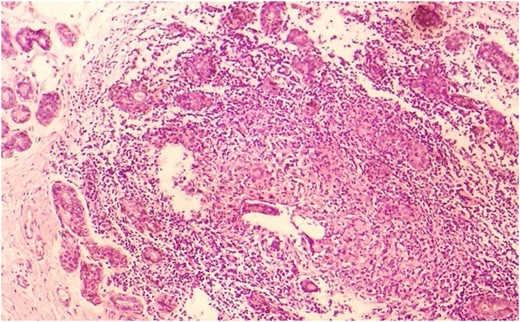
Our patient was a 37-year-old woman. Her aunt and her cousin has a breast cancer story. She was taking Levothyroxine 125 ug/day Levothyroxine by Hashimato's Thyroiditis diagnosis. She did not use OKS. Her gravity was 6, her parity was 2, her abortion was 3. During 14th week of her pregnancy, she applied to our clinics by rising rash, ache and swelling complaint (Fig. 1). In her breast ultrasonography, irregular limited solid and partly heterogeneous hypoechoic leisons (largest one's diameter 4 cm) and in right axilla a lymph node of 18.4 mm diameter was detected (Fig. 2). Serum leucocyte 14 900 mm3, C reactive protein (CRP) 76.2 mg/l, sedimentation 84 mm/h, prolactin 95.5 ng/ml, fibrinogen 460 mg/dl, D-dimer 1134 ug/l levels were high. CA 125; 38.8 U/ml and CA 15-3; 41.5 were high. Rheumatoid factor (RF), Homocysteine, vitamin B12, folic acid, ACE, thyroid hormones, nuclear antibody, anti-ds DNA antibody, anti-microsomal antibody, anti-thyroglobulin antibody values were normal. Blood type was 0 Rh (+). In genetic analysis for identifying D-dimer level, habitual abortion and IGM etiology, MTHFR-C 677 TT, β- fibrinogen-455G/A, PAİ-1 5 G/5 G, ACE İ/D pathologic gene polymorphisms were detected. In addition to this, prothrombin, factor V Leiden, factor XIII V34L, MTHFR A1298C, factor V H1299R, apolipoprotein (APO) B R3500Q, APO-E E3/E3 and mean platelet −1 a/a, BRCA 1 and BRCA 2 genes’ mutation were not detected. For dioristic diagnosis, tru-cut breast biopsy was conducted. IGM is diagnosed after histopathologic examination (Fig. 3). After breast abscess drainage, abscess gram staining and culture antibiograms were made. In, gram smear Gram (+) cocci is detected. In abscess culture, Escherichia coli reporoduced. Patient was given low-dose systemic (Prednol 4 mg/day/oral) and topical (0.1% betamethazone pomat) steroid treatment along with prolactin inhibitor (Cabergolin 1 mg/week/oral) for hyperprolactinemia for 4 weeks; and according to culture antibiogramme results she was given antibiotic (cefuroxime axetil 500 mg tablet 2 × 1). After treatment, high laboratory values returned to normal. Treatment follow-ups were established by hemogram and CRP. In 2 years follow-up no relapse occured.
DISCUSSION
Granulomatous mastitis (GM) was firstly determined by Kessler ve Wolloch in 1972 [1]. GM's have two kinds: primary and secondary (specific). Specific GM's arise from microorganisms such as corynebacterium, tuberculosis, blastomycosis and caseous necrosis reasons such as foreign body reaction and sarcoidosis [1].
IGM refers to idiopathic lobular mastit. It is detected in less than 1% of women who were applied breast biopsy . Generally seen in women in second-fourth decades and postnatally [1, 3]. It is rarely seen on men [1]. IGM is mostly seen in Asia and Mediterranean Region [1].
Etiology of IGM is not known definitively. For etiology, autoimmunity, hormonal disorders, race, cigarette, infectious factors, α1-antitrypsin deficiency, diabetes mellitus, breast trauma, obesity are researched [1]. In IGM treatment, activity of steroids supports autoimmunity. IGM can be seen singly or with sjögren syndrome, erythema nodosum, arthritis or thyroiditis which are autoimmune diseases [2]. Pathogenesis breast secretions and lymphocyte t-cell-mediated is explained with mediative autoimmune reaction [3]. However, since significant results were not obtained in vasculitis and autoimmune serologic tests, autoimmunity is argumentative. Close relationship is determined between IGM and pregnancy and lactation, OKS usage, hormonal disorders such as hyperprolactinemia [1, 2]. By hormonal effects such as oestrogen and prolactin increase, intraductal proteinosis secretion, ductal ectasia, intraductal inflammation and galactophoritis IGM development is noted [1]. In our pregnancy case, habitual abortion and Hashimato's thyroiditis story was seen.
Involvement in IGM is frequently unilateral. Bilateral involvement is seen 25% [1]. Painful breast mass and rash is seen in acute stage. Rarely, palpable lymph nodes can be detected in axilla. Mostly has chronical course; and confused with cancer in 50% of the cases by fistula, abscess, ulceration, nipple contraction and peau d'orange appearance [3, 4]. For IGM, in breast sonography irregular bounded, heterogen hypoechoic mass; in mammography focal asymmetric opacity, in breast MRG lesions, high contrast peripherals are seen generally. Radiologically there are no specific findings and can be similar to malignancy [2, 3]. For diagnosis gram staining and culture must be established; Ziehl–Neelsen and PAS staining must be established for differential diagnosis [2, 4]. Generally, in abscess culture, no reproduction occurs unless secondary infection such as corynebacteriums exist [1]. IGM diagnosis is made by exluding tuberculosis, fungal infection, sarcoidosis, of Wegener's granulomatosis and other specific granulomatous diseases such and breast carcinoma [1, 2]. IGM final diagnosis is established by needle aspiration biopsy, tru-cut or excisional biopsy. Histopathologically revealed lobular Noncaseating granuloma, giant cells, chronic inflammation is detected [4].
In our patient involvement in her right breast, right in the palpable axillary lymphadenopathy and abscesses culture of E. coli was detected. In genetic analysis of patient whose serum WBC, CRP, sedimentation, prolactin, fibrinogen, D-dimer levels were high, MTHFR CA 677 TT, β-fibrinogen-455 G>A, PAI-1 5 G/5 G, ACE I/D pathological gene polymorphisms were seen. Patient's CA 125 and CA 15-3 markers were high and brca1-2 gene analysis was normal. Final diagnosis was made by tru-cut biopsy breast examination.
There is no common treatment which is generally accepted for IGM [1]. In the treatment IGM, steroid therapy most widely applied. Other drugs used in the treatment are anti-inflammatories, colchicine and immunosuppressive agents such as methotrexate or azathioprine [1, 2]. In Medical treatment non-response, or clinical findings of recurrent abscess or fistula, wide local excision or even mastectomy can be applied [1, 4]. Recurrence rates can be up to 50%. The first 2 years of follow-up process is recommended in regular intervals of 3–6 months [2, 4]. Our patient was given treatment with breast abscess drainage, steroid, sensitive antibiotherapy and prolactin inhibitor. In 2 years follow-up no relapse occurred.
IGM can be confused with breast cancer [2, 3]. IGM and breast malignancy may occur together [5]. Some researchers specified that chronic inflammatory and hormonal effect in the breast are contributing to the formation of malignancies and may lead to particularly infiltrating breast carcinoma [5].
IGM, is a rare inflammatory breast disease although it is mostly accepted as an autoimmune disease [2]. In various publications, MTHFR-homozygous 677 TTR mutations [6], PAI-1 5 G/5 G polymorphism [7], β-fibrinogen heterozygous 455 G>A mutation [8], ACE I/D mutation [9] are reported as closely related with inflammatory, autoimmune events and breast cancer. In the studies, there seem to be a genetic predisposition in the development of many autoimmune and malignant diseases [10]. As seen in our case, various gene polymorphisms and induced inflammatory and autoimmune disorders may play a role in unknown IGM etiology.
RESULT
It is thought that IGM is a disease based on autoimmunity, hormonal disorders, etc. reasons. Clinical and radiological features are similar to breast carcinoma. Biopsy is required for final diagnosis. Various polymorphisms may play a role in IGM which is often accepted as an autoimmune disease. However, there is a need for more extensive scientific studies for.
CONFLICT OF INTEREST STATEMENT
None declare.
FUNDING
None.



