-
PDF
- Split View
-
Views
-
Cite
Cite
M. Kodeeswaran, Reshmi Udesh, L. Ramya, S. Jothi Kumar, Multiple cerebrospinal cavernous angiomas, Journal of Surgical Case Reports, Volume 2016, Issue 9, September 2016, rjw157, https://doi.org/10.1093/jscr/rjw157
Close - Share Icon Share
Abstract
Cavernous angiomas represent 5–12% of all spinal vascular lesions and 1% of all intramedullary lesions in pediatric patients. Intramedullary spinal cavernomas are relatively rare with only 24 cases reported till date to the best of our knowledge. A 15 -year-old boy presented to the clinic with acute onset motor weakness in all four limbs. He was diagnosed with multiple cerebral cavernomas and an acutely bleeding spinal cavernoma. Complete surgical excision of the spinal cavernoma was done. Postoperatively the patient's weakness gradually improved to a power of 4/5 in all his limbs over a period of 10 days. Only 24 cases of pediatric spinal cavernomas have been reported in the current literature. Current consensus on management of these rare lesions is based on previously published case reports/series and surgery appears to be the only definitive treatment. Further studies regarding any non-surgical expectant management appears warranted.
Introduction
Cavernous malformations (CMs) or cavernous angiomas are vascular malformations characterized by dilated closely apposed sinusoidal vessels lined by a thin layer of endothelium without any smooth muscle, elastin, adventitia or intervening neural parenchyma [1, 2]. They are low-flow vascular structures making them angiographically occult. Although they may localize in any part of the central nervous system, majority of the current literature reports cerebral or intracranial cavernomas with a prevelance rate ranging from 0.4% to 0.6% across both adult and pediatric populations [3]. Cavernous angiomas represent 5–12% of all spinal vascular lesions and 1% of all intramedullary lesions in pediatric patients [4]. Intramedullary spinal cavernomas are relatively rare with only 24 cases reported till date to the best of our knowledge [1, 4–8].
In this article, we describe our experience with a case of pediatric multiple cerebral and spinal cavernomas and review the current literature on the clinical characteristics, diagnosis and management of this rare condition.
Case Report
A 15-year-old adolescent boy presented to the clinic with history of urinary incontinence and progressive weakness in all four limbs over a period of 3 days. He had no history of previous similar episodes or previous neurological symptoms and no history of prior radiation therapy. Family history did not indicate any similar complaints. On examination the patient had motor weakness with a power of 3/5 and pyramidal tract signs in all four limbs, with Babinski's sign in both lower limbs. Signs of right hypoglossal nerve palsy such as wasting and fasciculation in the right half of the tongue and a distended bladder owing to urinary retention were also identified. The patient did not cooperate for a detailed sensory examination. He was admitted to the neurology ward for further evaluation. Within 24 hours of admission his symptoms worsened and he developed complete quadriplegia.
Magnetic resonance imaging (MRI) of the brain and spine showed multiple hypointense lesions in the brain and one mixed intense lesion with a hyperintense rim intrinsic to the spinal cord at the level of C5 with evidence of fresh bleeding (Figs 1–3). He was diagnosed with multiple cerebral cavernomas and an acutely bleeding spinal cavernoma. The patient was scheduled for a laminectomy and total excision of the spinal cavernoma and to control further hemorrhage. Bone removal was limited to the site of malformation. The lesion was identified by the bluish discoloration on the dorsal surface of the spinal cord (Fig. 4). A myelotomy was made over the discoloration and the lesion was excised inside out. Intraoperative evoked potential monitoring was not used for our procedure due to lack of availability.
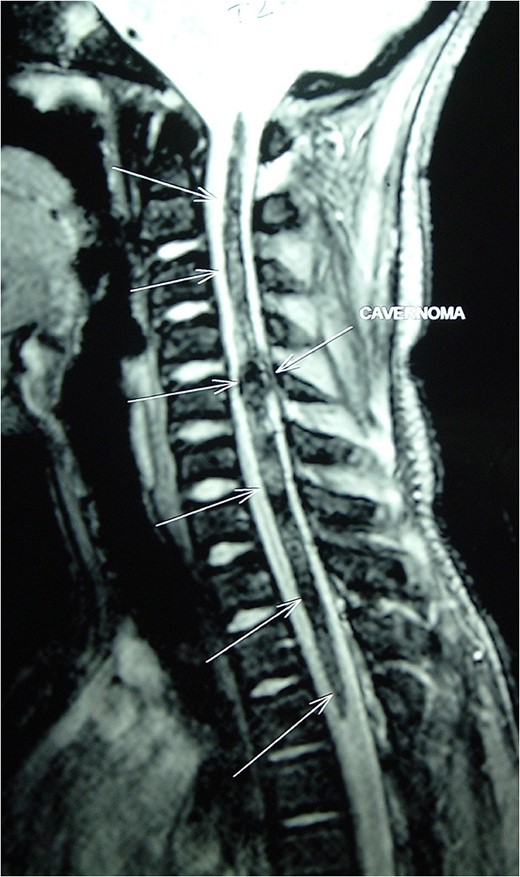
MRI Spine (GRE T2WI) sagittal view shows a localized lesion within the spinal cord at C5 with a mixed intensity focus centrally and a surrounding low-signal ring typical of cavernous angioma with surrounding hemosiderin deposition.
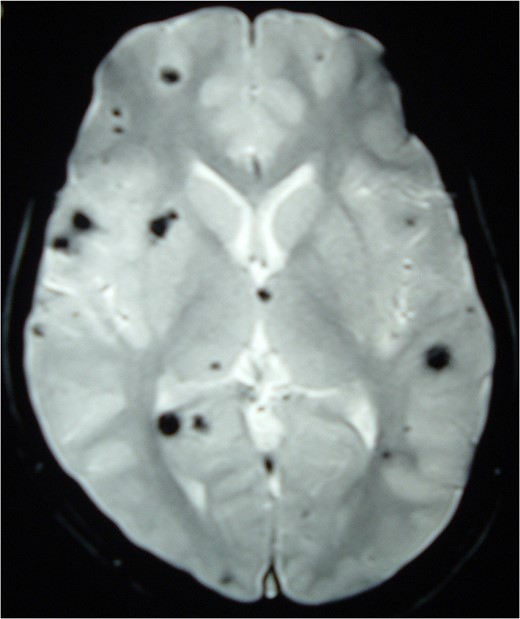
MRI Brain (GRE T2WI) showing multiple hypointense lesions diagnosed as cerebral cavernomas.
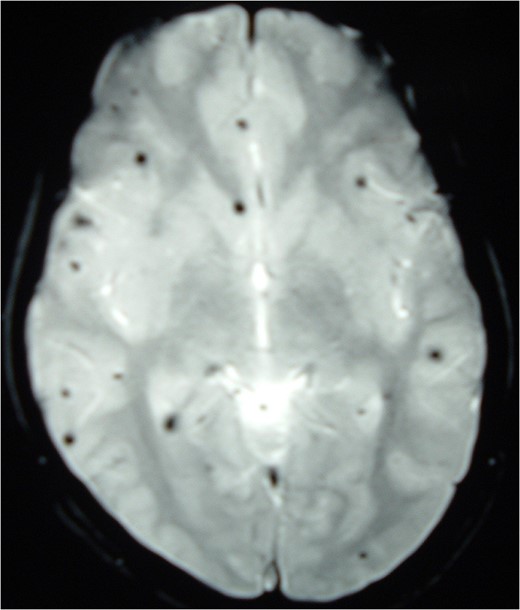
MRI Brain (GRE T2WI) showing multiple hypointense lesions diagnosed as cerebral cavernomas.
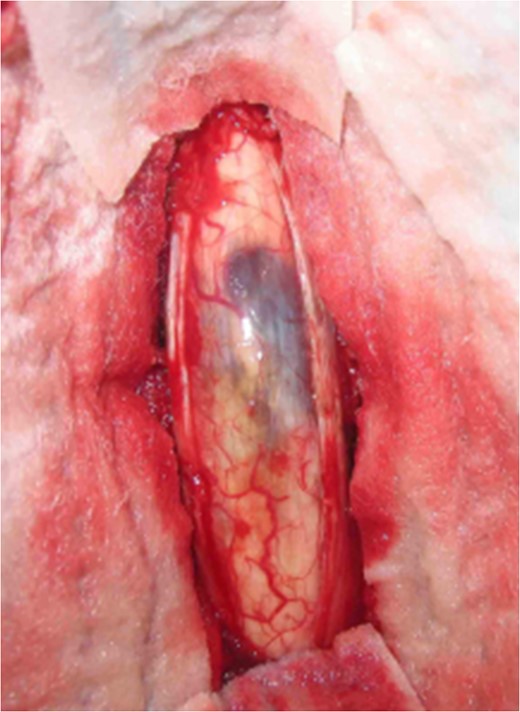
Intraoperative image at C5 showing subpial bluish discoloration at the spinal cord surface with underlying cavernous angioma.
The spinal cavernous angioma was completely excised and sent for histopathological examination (HPE). HPE (Figs 5 and 6) showed dilated vascular spaces lined by thinned out endothelium and fibrous adventitia without any evidence of neuronal tissue in between the vascular spaces thereby confirming the diagnosis of a cavernous angioma. Postoperatively the patient's weakness gradually improved to a power of 4/5 in all his limbs over a period of 10 days. The patient regained independent ambulation by 6–8 months. Immediate postoperative MRI showed complete resection of the cavernoma.
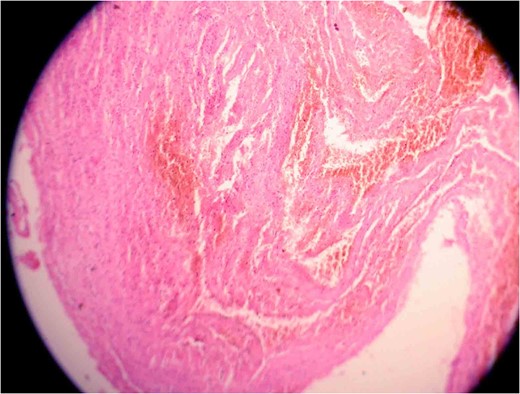
Photomicrograph one showing a vascular lesion composed of thin-walled, venous-type, congested blood vessels of various sizes closely packed together.
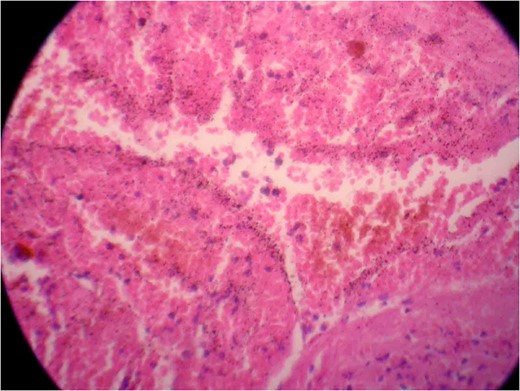
Photomicrograph two showing a vascular lesion composed of thin-walled, venous-type, congested blood vessels of various sizes closely packed together.
Discussion
Previous case series and retrospective studies report an age range of 5–15 years as the age at presentation for cavernomas with a predilection in males [1, 9]. Intramedullary cavernomas present predominantly in the thoracic spine in the adults. In children equal involvement of both cervical and thoracic regions is seen [1, 9]. The clinical presentation has been reported to follow one of the following courses distinct episodes of neurological decline with some recovery in between, gradual deterioration of neurological function, acute onset of neurological deficit with rapid worsening of symptoms or acute onset of mild symptoms with gradual decline [9, 10]. The last two courses appear to be the most common clinical scenarios in children [1, 4]. The acute onset of the myelopathy is attributed to spontaneous hemorrhage of the cavernoma which can have devastating neurological complications due to little room for expansion in the spinal cord [9, 10].
Intramedullary spinal cavernomas may be associated with multiple concurrent occult cerebral cavernomas as seen in our case. This makes MRI of the complete neuraxis a crucial next step [1, 4, 5]. Familial CM syndrome should be suspected for any case with multiple cerebral cavernomas [4]. MRI remains the gold standard for diagnosing both cerebral and intramedullary cavernomas. They appear as mixed signal intensity lesions in on T1 and T2-weighted images surrounded by a low-signal intensity zone (due to hemosiderin deposits) best seen on T2-weighted images [4, 5, 8].
Complete resection of the intramedullary lesion remains the general consensus to treat pediatric intramedullary cavernomas based on previous case reports [1, 4, 5, 9]. The lesion appears as a bluish discoloration on the dorsal surface of the spinal cord. Intraoperative ultrasound is used to localize the lesion and a myelotomy is done over the discolored region to completely resect the lesion [4, 9]. The gliotic plane surrounding the lesion is used as a demarcation to remove the lesion. Intraoperative electrophysiological monitoring with somatosensory evoked potentials and motor-evoked potentials may be used to monitor and prevent any impending perioperative neurological deficits [1, 9]. Histology of the resected lesion usually confirms the appearance of dilated thin-walled vascular structures lined by a single layer of endothelium without any intervening neural tissue [2, 9]. Postoperative MRI to confirm complete resection is desired [2].
Expectant management with frequent follow-up examinations and regular MRIs has been suggested in asymptomatic patients with incidental discovery of cavernomas and in patients with surgically inaccessible lesions [2, 9]. Due to the relative rarity of the condition any guidance for management we have is based on prior case series or case reports which uniformly dictate immediate surgical intervention due to the significant risk of morbidity associated with bleeding into a confined space. Long-term risks of laminectomy in pediatric cases such as spinal deformities have been suspected warranting regular follow-up [2, 9]. Further prospective studies exploring other management options and their long-term effects in pediatric spinal cavernomas could help us tailor treatment on a case by case basis.
Acknowledgements
None.
Conflict of interest statement
None declared.
Funding
None.



