-
PDF
- Split View
-
Views
-
Cite
Cite
Ryan C. Turner, Brandon P. Lucke-Wold, Roy Hwang, Bill D. Underwood, Lung cancer metastasis presenting as a solitary skull mass, Journal of Surgical Case Reports, Volume 2016, Issue 6, June 2016, rjw116, https://doi.org/10.1093/jscr/rjw116
Close - Share Icon Share
Abstract
Lung cancer has been well documented to spread to bone and the axial skeleton after metastasis to adjacent organs. Bony metastasis is not, however, the typical presenting manifestation. The differential diagnosis for a tissue mass on the skull should warrant a workup for metastatic disease. Bony metastasis plays an important role in treatment and disease management. We report an exceptionally rare case of stage IV lung adenocarcinoma that presented with a solitary skull metastasis and a significant soft-tissue component. The lesion was treated by excision via craniotomy and subsequent medical management of the adenocarcinoma. This case illustrates a very rare presentation of lung adenocarcinoma and also represents what the authors believe to be the first report of a solitary skull mass originating from a lung primary. We also present a review of the literature surrounding bony metastasis to the skull and implications for patient care.
INTRODUCTION
Bony metastases are a common finding in numerous cancers, particularly breast, prostate and lung. Lung cancer specifically is associated with bony metastases in up to 36% of patients on post-mortem studies [1]. Similarly, bony metastases are the most common cause of cancer-related pain and can be utilized as a marker for monitoring disease progression and prognostication [1, 2]. It is the pain, often associated with pathological fracture that often leads patients to seek medical treatment in the case of bony metastases. Generally a bony metastasis by itself is unlikely to lead to medical presentation and eventual diagnosis of the primary malignancy. This is especially true when the metastasis is located within the skull and can be mistaken for soft tissue swelling from injury. In fact, Sugiura et al. [2] identified skull metastases in only 3% of skeletal metastases from lung cancer. Likewise, no evidence in the literature describes a solitary skull metastasis as the primary presenting feature for lung adenocarcinoma.
In this report, we present the case of a 69-year-old female that presented to the clinic complaining of an expanding soft-tissue mass on her left frontoparietal skull associated with facial pain. The patient received a complete oncological workup and surgical excision of the mass. The excised mass was pathologically consistent with metastatic lung adenocarcinoma. This is the first such presentation of primary lung adenocarcinoma metastasis to the skull in the literature and dictates caution and careful clinical consideration when evaluating expanding lesions on the skull. This is especially important for lung adenocarcinoma where early diagnosis can affect overall prognosis.
CASE REPORT
The patient, a 69-year-old white female, was referred by her primary care provider to a plastic surgeon at United Hospital Center for treatment of what was believed to be a soft-tissue mass of the left forehead (frontoparietal region) (Fig. 1). The mass had increased in size for the prior 2–3 months and resulted in a radiating pain down the left side of the face. The mass was non-mobile and tender to touch. Prior to this referral, the patient was relatively healthy with a past medical history significant for arthritis, depression and hypertension. Her surgical history was positive for tonsillectomy and partial hysterectomy. Her social history is significant for a 60-pack-year smoking history (1.5 packs/day for 40 years). The soft tissue mass had been managed conservatively for presumptive dermatological infection (subcutaneous/sebaceous cyst) with a 10-day course of levofloxacin 500 mg tablets taken once daily. The tablets, however, provided no symptomatic relief. Imaging (computed tomography (CT)) of the maxillofacial area and brain without contrast was obtained in addition to plain films of the skull. These images revealed a lytic lesion of the skull in the left frontopartietal region (Fig. 2). The patient was referred to the oncology department for further workup.
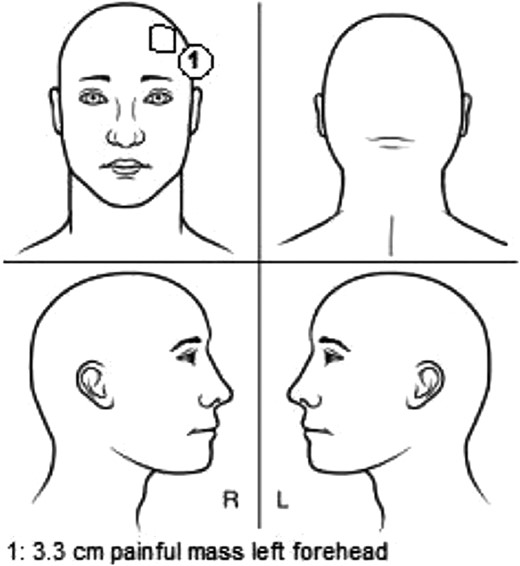
Schematic representation of the lesion location. The soft-tissue component was measured at a diameter of 3.3 cm and located on the left forehead near the hairline.
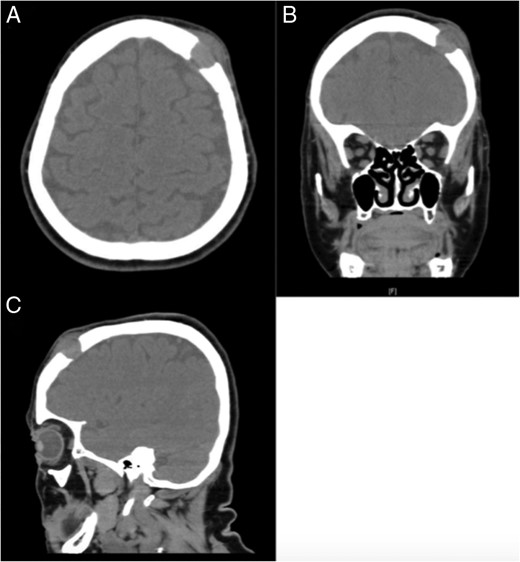
CT soft tissue demonstrating the presence of a lytic lesion in the left frontopartietal region. (A) Axial cut, (B) coronal cut and (C) sagittal cut.
The primary differential considered by the oncologist based on the significant smoking history was metastatic neoplasm versus multiple myeloma. Therefore, a CT of the chest/abdomen/pelvis was ordered as well as blood work (CBC, chemistry, molecular monoclonal gammopathy workup). Imaging demonstrated one speculated right hilar nodule concerning for neoplasm in the lung that was measured at 2.4 × 2.4 cm. No other evidence of metastatic disease was present within the chest, abdomen or pelvis. Workup for multiple myeloma was negative based on the results of serum protein electrophoresis and immunofixation. The patient was subsequently referred for bronchoscopy and to neurosurgery for biopsy of the skull mass.
Neurosurgical examination revealed grossly intact cranial nerves with no upper motor neuron signs or motor weakness in all extremities. Sensation was also intact bilaterally. Due to slight effacement of the subarachnoid space and for surgical planning, MRI brain was ordered. MRI demonstrated a 2.8-cm lesion that extended from the subcutaneous fat through the inner and the outer table of the left frontal bone with slight encroachment on the dural space. Minimal mass effect was observed with only slight asymmetry of the fluid signal in the frontoparietal region on the left (Fig. 3). At this time, the decision was made to proceed with a surgical excision. No apparent invasion of the dura or brain parenchyma was noted, thereby satisfying therapeutic and diagnostic aims. The lesion was excised successfully (3.5 × 3.5 × 1.5 cm) with no surgical complications. Histological analysis of the lesion revealed adenocarcinoma that was CK7 positive, CK20 negative and TTF-1 positive. These findings were consistent with a metastatic adenocarcinoma from a lung primary. With stage IV adenocarcinoma, the patient was started on chemotherapy following surgical excision and managed by the oncology service. Her management included a full oncological workup complete with full-body PET scan.
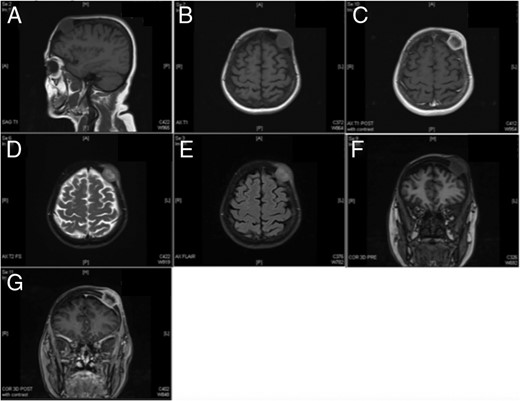
MR imaging redemonstrating the presence of a left frontoparietal mass that does not appear to invade the dura. (A) Sagittal T1, (B) axial T1, (C) axial T1 with contrast, (D) axial T2, (E) axial FLAIR, (F) coronal pre-contrast and (G) coronal post-contrast.
DISCUSSION
While the rate of bony metastases associated with lung cancer is relatively high, estimated at ~36%, only 3% of these are generally found within the skull. The first presenting sign of lung adenocarcinoma being skull metastasis is exceptionally rare and has not been previously reported in the literature. This case provides an example of such findings. This is in contrast to other malignancies, such as hepatocellular carcinoma (HCC), which has been widely reported to present as a solitary skull metastsis [3]. Hepatic cholangiocarcinoma, in particular, is exceptionally prone to metastasize to the skull [4]. Therefore, it is clear for diagnostic, therapeutic and prognostic purposes that a biopsy must be obtained, which was excisional in nature in the reported case.
The route of metastasis in this case is not clear but possible routes have been discussed at length in the literature and include transmission from mandibular lymphatics. Once seeded, the tumor develops a rich vasculature supply and spreads into adjacent soft-tissue [5]. In rare cases, skull metastasis can lead to epidural hematoma [6]. The important component for treatment is TNM staging. CT scans have become the gold standard for diagnosis and staging of lung adenocarcinoma [7]. Evidence suggests that CT scan is even better than radionucleotide imaging for skull metastasis [8]. With regard to treatment, the initial extent of the disease dictates overall survival [9]. Clinical suspicion should be high when evaluating an isolated soft-tissue lesion with skull penetration. Skull biopsy with tumor excision followed by aggressive chemotherapy is often required [10]. Sugiura et al. [2] found that in women with lung adenocarcinoma and bony metastasis that early initiation of systemic chemotherapy was associated with a good overall survival and prognosis.
Based on the findings from this case, the authors recommend a careful and thorough evaluation for any persistent soft-tissue mass on the skull. Early diagnosis and treatment can lead to good overall prognosis. Clinical suspicion should be high for this potentially life-threatening disease.
Conflict of interest statement
None declared.
FUNDING
A Neurosurgery Research Funding and Development Grant, American Medical Association Foundation Seed Grant, and American Foundation of Pharmaceutical Education Pre-doctoral Fellowship supported Brandon Lucke-Wold.



