-
PDF
- Split View
-
Views
-
Cite
Cite
Rose Park, Kira Vincent, Abd A. Abdelrahman, Mridula Krishnan, Jahnavi Koppala, An unusual presentation of recurring metastatic melanoma, Journal of Surgical Case Reports, Volume 2016, Issue 3, March 2016, rjw030, https://doi.org/10.1093/jscr/rjw030
Close - Share Icon Share
Abstract
A 53-year-old non-distressed Caucasian female complains of dyspnea and palpitations for 5 days. Past medical history includes Stage IV melanoma with adequate resection 23 years prior. The patient suddenly became increasingly tachycardic in mild respiratory distress while maintaining hemodynamic stability. TTE depicted 10.5 × 7.5 × 9.5 cm3 mass within her left ventricle and a large volume of pericardial effusion, which progressed to cardiac tamponade. Pericardial window was performed. Metastatic involvement should be ruled out for all symptomatic patients with a history of melanoma.
INTRODUCTION
Melanoma remains a public health concern, as incidence continues to rise with mortality remaining stable [1].
CASE REPORT
A 53-year-old Caucasian female was diagnosed with malignant melanoma of the superficial spreading variant on the right shoulder in 1992; the tumor, originally 4 cm in diameter, was considered adequately excised along the length of her right shoulder with 3 mm maximal depth.
The patient presented to the Emergency Department complaining of sudden, worsening shortness of breath and palpitations for 5 days. She denied any chest pain, cough, orthopnea or paroxysmal nocturnal dyspnea. On examination, the patient was alert, oriented and in mild respiratory distress. Her blood pressure was 123/70 and pulse at 115 beats per minute with respiratory rate at 25 breaths per minute with oxygen saturations at 95% on room air. Examination of her cardiac field demonstrated regular tachycardia with diminished heart sounds.
Electrocardiogram (ECG) documented upon arrival showed sinus tachycardia with diffuse, low-voltage QRS complexes. Blood workup, which included complete blood count, comprehensive metabolic profile, and troponin levels, was within normal limits.
Chest X-ray found an enlarged cardiomediastinal silhouette with mild pulmonary edema (Fig. 1). A computed tomography angiography (CTA) of the chest to rule out pulmonary embolism (PE) was ordered. After the patient returned from imaging, she was found to have a heart rate of 178. Patient was awake, did not appear to be in any more distress and remained hemodynamically stable. Repeated ECG showed monomorphic ventricular tachycardia, with the patient remaining non-distressed. Patient was started on i.v. amiodarone, and urgent cardiology consult was obtained. A transthoracic echocardiogram depicted a large mass measuring 5 cm by 7 cm occupying the basal and mid portion of the anterior and lateral walls of the left ventricles with large pericardial effusion located at the lateral posterior wall of the heart, findings suggestive of impending tamponade (Fig. 2). CTA of the chest reported no acute PE with a large mass (10.5 × 7.5 × 9.5 cm3) occupying the anterior wall of the left ventricle and extending to the pulmonary outflow tract (Fig. 3).
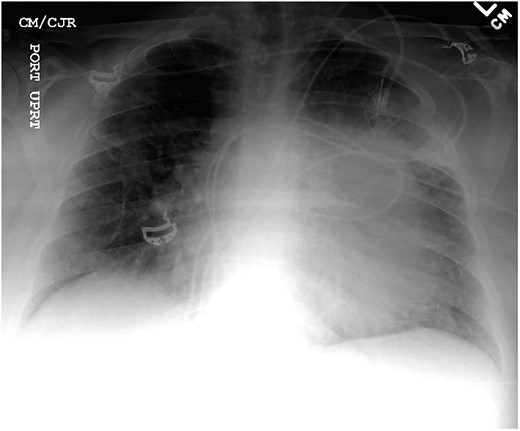
Anterior–posterior one view chest X-ray depicting enlarged cardiomediastinal silhouette with mild pulmonary edema.
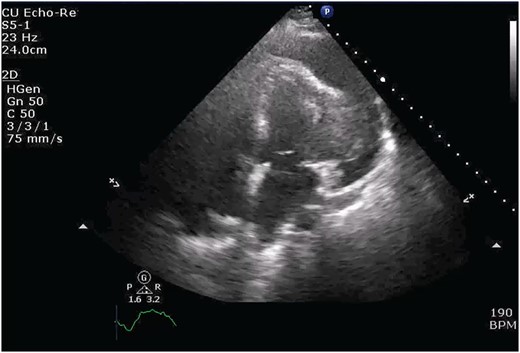
Transthoracic echocardiogram depicting large effusion within pericardial membrane with left ventricular mass.
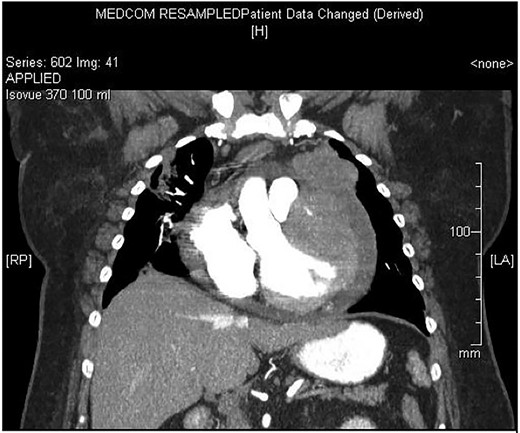
Large ventricular mass measuring 10.5 × 7.5 × 9.5 cm3 invading epicardium and myocardium.
Cardiothoracic surgery team was consulted, and the patient underwent a pericardial window with 750 cc of serosanguinous fluid drained from her pericardial space. A chest tube placed status-post pericardial window, which initially continued to drain 500 cc of serosanguinous fluid, with fluid volume decreasing in the following 5 days with stable vital signs. The patient tolerated the switch to p.o. amiodarone and discharged upon her request to restart chemotherapy, after removal of her chest tube. Histopathology results of the pericardial fluid were positive for reactive mesothelial cells and chronic inflammation. Follow-up positron emission tomography (PET)/CT scan depicted increased uptake in the cardiac chamber (Fig. 4). Repeated echocardiography 2 months later depicted a return in pericardial fluid with the patient remaining hemodynamically stable with preserved ejection fraction.
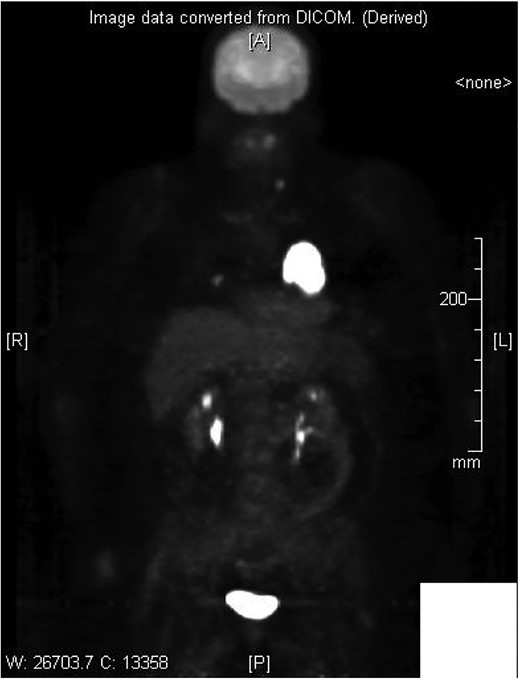
DISCUSSION
To better stage malignant melanomas, Breslow first established a rating system in 1970 to estimate prognoses [2]. His staging system, based on thickness of metastatic tumor and depth of invasion, continues to be of value in assessing prognosis [2]. Our patient, having received a Breslow depth of 3 mm, qualified for a Stage IV; Stage IV 5-year survival rates are 15–20% [3]. Nearly one-third of all patients diagnosed with metastatic melanoma will experience reoccurrence of their disease [4].
Metastatic melanomas are considered common neoplasms having the propensity to metastasize to a multitude of regions, including the heart. For patients with a history of malignant melanomas, up to 71% of them can have some cardiac metastatic involvement, while only 10% of them are actually symptomatic [5]. Within the heart, the right atrium is most commonly involved for cardiac metastases [6]. Our patient had no right atrium findings; the mass had invaded both the epicardium and myocardium of the left ventricle. In addition, the patient continued to remain hemodynamically stable despite the large metastatic mass present within her left ventricle.
For patients with cardiac metastatic melanoma involvement, hemorrhagic pericardial effusions are often the first presentations of such involvement [7]. Utilizing ECGs to detect cardiac involvement may prove futile; while patients with normal ECGs were less likely to have cardiac metastases, nonspecific ST-T wave changes were difficult in differentiating involvement [8]. The typical presentation of cardiac tamponade is hypotension, pulsus paradoxus >12 mmHg and elevated jugular venous pressures, or alternate-beat variation in the beat, direction, amplitude and duration of a component of the ECG. For our patient, there was difficulty in appreciating ECG findings such as low-voltage or eletricans alternans due to her ventricular tachycardia.
For patients with a history of melanoma, metastatic involvement should be ruled out for any cardiac conditions. The unique aspect of our case is the length of asymptomatic metastatic growth. For patients with a history of Stage IV malignant melanoma, close follow-up should be thoroughly maintained, regardless of symptomatology, to determine if there are any metastases. ECG may not be a reliable source to rule out pericardial involvement as such findings are nonspecific and unreliable and should therefore not be used as the only test to exclude cardiac involvement. Follow-up should be continued thereafter to routinely rule out asymptomatic metastases.
CONFLICT OF INTEREST STATEMENT
None declared.
ACKNOWLEDGEMENTS
We wish to acknowledge Dr Jeffrey Sugimoto M.D. and his surgical expertise.



