-
PDF
- Split View
-
Views
-
Cite
Cite
Samuel T.W. Lo, Siu Lan Leung, Chung Ngai Tang, Abdominal wall necrotising fasciitis secondary to fish bone ingestion, Journal of Surgical Case Reports, Volume 2015, Issue 7, July 2015, rjv078, https://doi.org/10.1093/jscr/rjv078
Close - Share Icon Share
Abstract
Abdominal wall necrotising fasciitis secondary to fish bone ingestion is extremely rare. We present a case of ingested fish bone complicated with self-sealing small bowel perforation and abdominal wall necrotising fasciitis. Following principles of necrotising fasciitis, a high index of suspicion led to early diagnosis and early treatment. The patient enjoyed a good recovery.
INTRODUCTION
Fish bone ingestion is common in both adults and children. The majority of ingested foreign bodies can be retrieved or pass through the gastrointestinal tract uneventfully. Less than 1% of patients with foreign body ingestion develop gastrointestinal perforation. In our case, the small bowel perforation was self-sealing, but the ingested fish bone also caused necrotising fasciitis of the abdominal wall.
CASE REPORT
A 63-year-old lady with good past health presented to our hospital complaining of right-sided abdominal pain for 3 days. There were no associated symptoms. On physical examination, she was afebrile with stable vital signs. There was right abdominal patchy erythema from the level of the lower ribs down to, and including, the vulva. Right vulval mild swelling was present. Palpation revealed right abdominal tenderness. There was no crepitus or subcutaneous emphysema.
Chest X-ray showed no pneumoperitoneum. Blood tests revealed leucocytosis. A contrast computed tomography (CT) scan showed a gas-containing collection at the right abdominal wall with a linear hyperdensity visible in the abdomen (Fig. 1). There was no pneumoperitoneum nor obvious intra-abdominal organ injury.
Debridement was performed within a few hours. Laparoscopy showed that there was a fish bone that had perforated through the small bowel and lodged in the abdominal wall causing an abscess to form (Fig. 2). The small bowel perforation had spontaneously sealed off, and the infection had travelled down along the fascia down to the vulva.
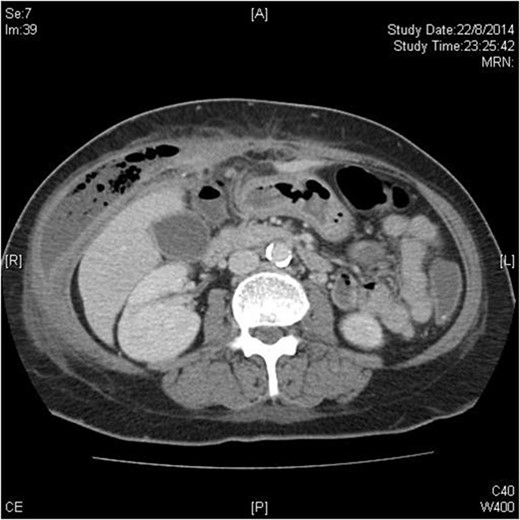
Transverse plane of CT scan showing right-sided abdominal wall collection with fish bone just visible as a hyperdense dot just anterior to the liver.
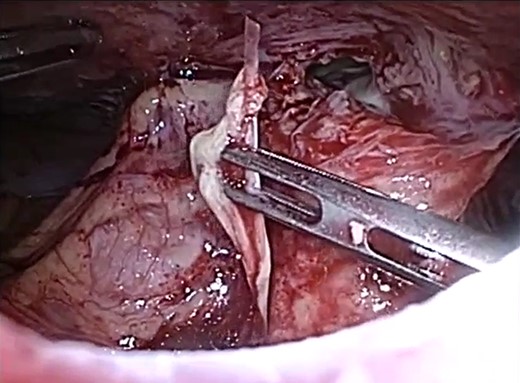
Laparoscopic view of the abdominal wall abscess and retrieved fish bone.
She required three debridements. Laparoscopy during each debridement confirmed no peritonitis or bowel injury. The only sub-fascial defect was 2 cm in size and was repaired. The resulting abdominal wall defect was in excess of 50 × 20 cm (Fig. 3).
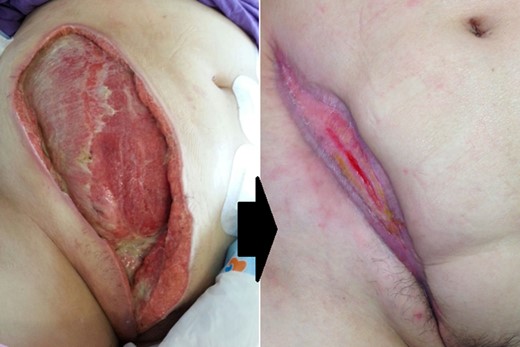
Abdominal wall defect 1 day after the third debridement and the wound healing after 20 weeks.
Between the debridements, she had persistent sepsis and developed cardiovascular complications; takotsubo's cardiomyopathy, supraventricular tachycardia and pulseless ventricular tachycardia (VT) requiring defibrillation, with return of spontaneous circulation.
However, she recovered well. The wound was managed with wound lavage and vacuum dressing. The wound healed primarily. She was discharged from hospital on post-op day 32. She was put on a wound empowerment programme by the wound team. The wound had almost completely healed after 5 months. She is continued to be followed up by our wound and surgical team.
DISCUSSION
Necrotising fasciitis is a severe infection of the subcutaneous fascia. Although it can occur anywhere, it is most common in the lower limbs, followed by the abdominal wall and perineum. The mortality rate without treatment approaches 100% [1]. Early diagnosis and early treatment play a crucial role in saving lives of victims with necrotising fasciitis.
The aetiology of abdominal wall necrotising fasciitis can be external or internal. External causes include trauma, which can be minor or major, ranging from mosquito bites to blunt trauma, and also surgical wounds. Internal causes are mostly secondary to intra-abdominal sepsis such as perforated appendicitis, gangrenous cholecystitis and bowel perforation.
Necrotising fasciitis is infection of the superficial fascia. Anaerobic bacteria grow and produce enzymes that destroy the fascia and fat. Nutrient vessels in the hypodermis are thrombosed, leading to tissue ischaemia, worsened by oedema. In turn, tissue ischaemia leads to infectious dissemination and skin necrosis [2].
It has been proposed to divide necrotising fasciitis into four types according to their microbiology. Type I is polymicrobial. Type II is monomicrobial infection with beta-haemolytic Streptococcus A [Streptococcus pyogenes]. Type III is monomicrobial infection with Clostridium species or Gram-negative bacteria such as Vibrios species or Aeromonas. Type IV is by fungal infections [3].
The diagnosis of necrotising fasciitis can be difficult, and a high index of suspicion is required in the early phase before sepsis becomes overwhelming. Early clinical signs and symptoms may only suggest local pain, erythema and swelling. As for investigations, blood tests are often non-specific. The ‘finger test’, involving a 2-cm incision down to the deep fascia followed by gentle probing of a finger, may yield ‘dishwater pus’, lack of bleeding and lack of tissue resistance to blunt finger dissection [2].
CT and magnetic resonance imaging (MRI) can help to establish the diagnosis by showing tissue infection, fascial swelling and inflammation. It can also demonstrate gas in the soft tissue, which is present in almost half of all patients [2].
Emergency surgical debridement of the affected tissues is the primary management modality for necrotising fasciitis. Multiple debridements are often needed [2]. The skin incision should be performed in a longitudinal direction along the muscle–fascial layers of the inner-abdominal wall until healthy fascia is found. Parallel or ventricle incision will result in bridges of skin, and skin islands will not usually survive. Extension of the infection to involve the bowel or the peritoneum is not uncommon and may require laparotomy [4].
Postoperatively, abdominal wall wounds require serial dressing changes until the wound is free of infection. Vacuum-assisted wound-closing device can be useful. The primary abdominal wall defect is usually large. Repair may require advanced flaps using an abdominoplasty technique, biological mesh or skin grafts [4]. Antimicrobial therapy is important but insufficient without surgical debridement.
There are a few factors that make this case noteworthy. The first is the rarity. The second is the early diagnosis and early treatment; the diagnosis was made within hours of admission, and the first debridement was performed soon afterwards, without delay. The third is the good outcome that the patient survived without morbidity and also without requiring abdominoplasty.
CONFLICT OF INTEREST STATEMENT
None declared.



