-
PDF
- Split View
-
Views
-
Cite
Cite
Zak Vinnicombe, Max Little, Andrew Wan, Laparo-endoscopic combined approach for excision of gastrointestinal stromal tumour close to the oesophagogastric junction, Journal of Surgical Case Reports, Volume 2015, Issue 7, July 2015, rjv079, https://doi.org/10.1093/jscr/rjv079
Close - Share Icon Share
Abstract
Tumours close to the oesophagogastric junction (OGJ) are difficult to excise due to high risk of complications. Laparoscopic and endoscopic combined surgery allows minimally invasive access while increasing tumour visualization. Computed tomography (CT) scanning of a 68-year-old female demonstrated a lesion suspicious of a gastrointestinal stromal tumour located 2 cm from the OGJ on the posterior gastric wall. Stapled excision was performed intragastrically and followed by endoscopic removal. Gastroscopy 7 months post-op and follow-up CT scan at 5 years demonstrated no recurrence of the primary tumour and no new disease. Laparoscopic and endoscopic combined approach is a safe and effective method of removing tumours close to the OGJ.
INTRODUCTION
Surgical resection provides the best opportunity for a positive outcome for patients with gastrointestinal stromal tumours (GISTs), with a minimally invasive approach improving postoperative recovery.
Tumour location within the stomach, particularly proximity to the oesophagogastric junction (OGJ), is often cited as a reason for a particular surgical technique, including ‘exogastric’, ‘transgastric’ and ‘intragastric’ approaches [1–3]. Tumours located close to the OGJ are particularly difficult to excise because of the high risk of causing strictures or leakage at the site of resection [1].
A laparoscopic and endoscopic combination surgery (LECS) for tumours of the cardia or those in close proximity to the OGJ has been described in several small studies [2, 4]. This combined approach is employed to obtain minimally invasive access and increase tumour visualization.
We describe utilizing this novel technique in the treatment of a patient with a GIST located high in the cardia, close to the OGJ.
CASE REPORT
A 68-year-old female underwent a computed tomography (CT) scan of her thorax and abdomen due to the presence of a RET oncogene variant and a history of recurrent tubulovillous polyps of the large bowel. The CT scan showed a 3.7 cm enhancing ‘polyp’ in the lumen of the stomach (Fig. 1) and large hiatus hernia with dilation of the lower oesophagus. Subsequent endoscopic ultrasound showed a lesion suspicious for a GIST located within 2 cm of the OGJ.
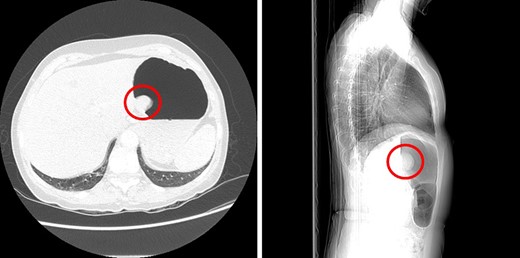
Sagittal and axial CT images showing a large polyp on the posterior gastric wall.
The decision was made to surgically remove the tumour and perform concurrent hiatus hernia repair. The tumour's proximity to the OGJ and current evidence suggested that LECS would provide an improved outcome compared with a more radical resection.
The patient was placed under general anaesthetic, optical access was acquired via the left upper quadrant of the abdomen using a 12-mm Xcel® (Ethicon) trocar and CO2 pneumoperitoneum was created. Two 5-mm Xcel® (Ethicon) trocars, one 12-mm Xcel® (Ethicon) trocar and a Nathanson liver retractor (Fig. 2A) were inserted under direct vision.
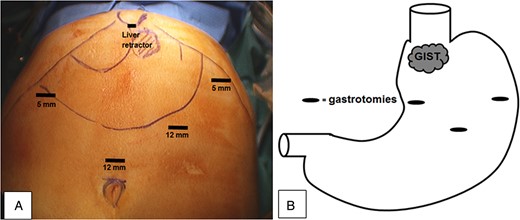
(A) Instrument access points and (B) gastrotomy positions on the anterior stomach wall and GIST location close to the OGJ.
A balloon trocar (Covidien) was inserted and inflated through a small incision in the anterior stomach, allowing the stomach to be elevated to the anterior abdominal wall. Gastrotomy positions for subsequent instruments are shown in Fig. 2B.
Endoscopic assistance allowed intermittent insufflation of the stomach to maintain an adequate surgical field and also aided in location of the OGJ. The tumour was directly visualized—a 3.5 cm × 2 cm sub-mucosal, pedunculated mass on the posterior wall, close to the OGJ.
The tumour was lifted away from the posterior wall with the grasper and a stapled excision performed using an endoGIA™ (Covidien) (Fig. 3A). The tumour was placed into an endobag™ and removed endoscopically, and the gastrotomies were closed with 2-0 vicryl. A crural repair and a partial fundoplication were completed with 2-0 ethibond™.
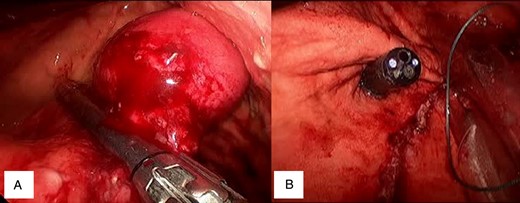
(A) Tumour elevation and stapler placement. (B) Staple line proximity to OGJ.
Histology showed low-grade GIST, categorized via National Institutes of Health (NIH) risk table [5]. The tumour was <5 cm, and there were <6 mitoses per 50 high-powered fields.
Operative time was 90 min, and there were no immediate postoperative complications.
Gastroscopy 7 months post-op demonstrated no recurrence of the primary tumour. Follow-up CT scan 5 years post-op showed no recurrence of tumour.
DISCUSSION
The primary aims of the surgery are to remove the GIST with negative resection margins and to preserve anatomical function. Tumour characteristics including size, type of growth (i.e. endophytic or exophytic) and distance from the OGJ are often cited as indications for a particular surgical technique [6].
Ohashi [7] described a new technique for treatment of gastric cancers in which laparoscopic instruments were passed into the stomach and all resection completed intraluminally. Subsequently, authors have reported a number of variations on this technique.
Posterior wall tumours showing predominantly endophytic growth can be resected using a laparoscopic, transgastric technique through gastrotomy in the anterior wall [1]. This is advantageous because it permits direct visualization of the lesion and better control of the surgical margin [8]. However, gastrotomy is associated with intraperitoneal spillage of gastric contents and bleeding from the gastric wall. Intraoperative tumour rupture during a transgastric procedure also carries the risk of peritoneal seeding.
A combined laparoscopic–endoscopic approach has been utilized in a number of patients with tumours close to the OGJ [1, 2, 9], but there is a paucity of literature in this area (particularly in the UK) on details such as success rates, complications and follow-up. Taniguchi et al. [10] reported a case study of a patient undergoing successful LECS of a tumour located in the cardia. More recently, Shim et al. [1] reported a series of six patients with tumours close to the OGJ. They concluded that this hybrid technique was safe, technically feasible and useful for resecting tumours close to the OGJ. A number of other case reports and small studies support these conclusions [2, 4].
Current literature suggests a number of advantages to this technique. Decreased deformity or stenosis of the OGJ, precise tumour location, determination of stomach port sites, confirming haemostasis at the staple line, retrieval of tumour and checking for air leakage post-resection have all been suggested as likely advantages of LECS [2, 9]. Furthermore, endo-linear stapler usage minimizes potential blood loss during tumour resection, decreases operation time and requires no suturing of resected stomach wall.
In this case, decreased peritoneal seeding of the tumour and decreased fracturing during resection, together with visualized port placement and accurate location of the OGJ, were the primary reasons behind selecting LECS.
This case study demonstrates that LECS is a safe and effective method of treating tumours close to the OGJ. The restriction of anatomical damage and more conservative surgical nature of the LECS are of particular importance for both short- and long-term patient recoveries. The flexibility of instrument placement also allows removal of the GIST with concurrent repair of a small hiatus hernia.
The 7-month and 4-year follow-ups have highlighted no short- or long-term sequelae, adding to the growing body of literature supporting this surgical approach. However, due to the recent developments in this area, larger studies are needed to confirm technical efficacy and longer-term patient outcomes.
CONFLICT OF INTEREST STATEMENT
None declared.



