-
PDF
- Split View
-
Views
-
Cite
Cite
Emma Illingworth, Paul S. Rooney, Richard Heath, Coonoor R. Chandrasekar, The use of a biological graft for the closure of large abdominal wall defects following excision of soft tissue tumours, Journal of Surgical Case Reports, Volume 2015, Issue 6, June 2015, rjv063, https://doi.org/10.1093/jscr/rjv063
Close - Share Icon Share
Abstract
Primary soft tissue tumours arising from the abdominal wall are uncommon and surgical excision of such tumours can result in large abdominal wall defects. There are many techniques available for abdominal wall repair following tumour excision, each having its own advantages and disadvantages. The options range from direct closure to the use of tissue flap reconstructions and/or prosthetic meshes. Currently, synthetic material such as polypropylene mesh is a common choice for closure of abdominal wall defects after tumour excision. Biological meshes are an alternative option for repair, and this report outlines two cases of abdominal wall repair using the porcine intestinal submucosa biological graft following excision of abdominal wall tumours. There was no evidence of infection, recurrence, seroma or hernias at 2-year follow-up. Following excision of soft tissue tumours of the abdominal wall, biological reconstructions can be successfully used to bridge the defect with minimal morbidity.
INTRODUCTION
Permanent synthetic meshes composed of materials such as polypropylene, polyester or expanded polytetrafluoroethylene are widely regarded as durable and ergonomically sound options for abdominal wall reconstruction [1, 2]. However, significant complications including mesh migration, bacterial colonization and fistula formation necessitated the development of an alternative [3]. The use of synthetic meshes can cause of adhesions, chronic sinus (2–6%), fistula formation (0–2%) and wound infections (2–17%). The development of biological grafts reduced the incidence of the aforementioned complications [3, 4]. Today, an array of grafts is available, including human, bovine and porcine dermis, as well as porcine small intestinal submucosa and bovine pericardium [5]. The following cases concern the successful repair of abdominal wall defects using the Biodesign® (Cook Medical™) porcine small intestinal submucosal (non-cross-linked) graft. Both of the procedures described were primary surgeries for large defects secondary to soft tissue tumour excision, an application of the porcine intestinal biological graft which is not yet widely documented in the literature.
CASE REPORT
Case 1
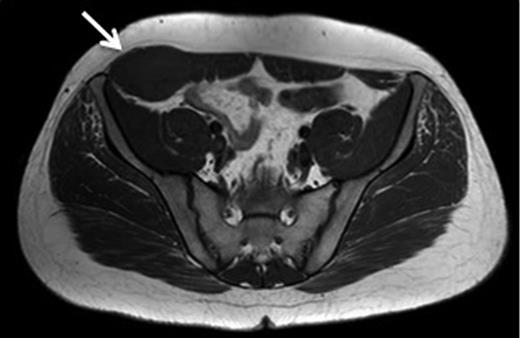
A 43-year-old Caucasian female presented to her General Practitioner in April 2012 with a 2-year history of a firm, painful swelling in the right flank. MRI revealed a 5.0 × 6.0 × 7.0 cm enhancing lesion with areas of necrosis, which was invading the antero-lateral abdominal wall (Fig. 1 ). With radiological features highly suggestive of a sarcoma, she underwent an ultrasound-guided biopsy, which classified the mass as a borderline myoepithelial tumour. The patient underwent an uncomplicated resection of the mass, which left a 10 × 10 cm right-sided antero-lateral abdominal wall defect. The defect was repaired using a Biodesign® biological graft. Initially, a layer of the biological mesh was used to cover the intact peritoneum with attachments cranially to the ribs and inferiorly to the right iliac crest. The external oblique was mobilized to partially cover the mesh, and a further layer of the biological mesh attached over it with Ethilon™ (Fig. 2). The patient had an uncomplicated postoperative recovery. The tumour histology revealed a 6.5 × 6.0 × 5.5 cm myxoid mass; immunohistochemistry analysis favoured a benign/borderline myofibroblastic tumour. At 24-month follow-up, she had good wound healing with a small area of paraesthesia inferior to the scar. An MRI showed good graft incorporation and no evidence of disease recurrence or hernia (Fig. 3).
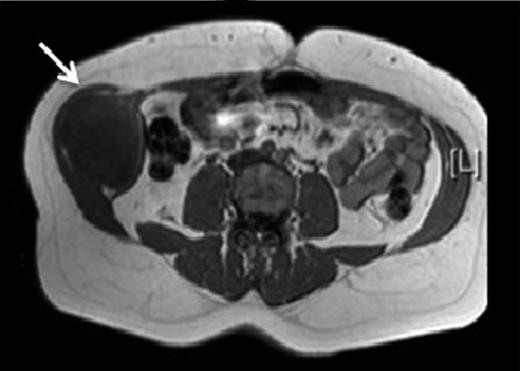
T1 MRI showing a soft tissue abdominal wall tumour involving external oblique, internal oblique and transversus abdominis and not involving the peritoneum (arrow).
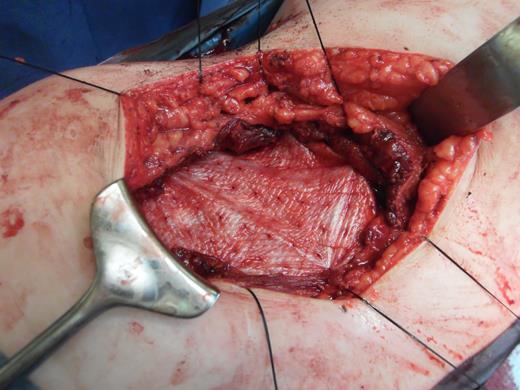
Depicting the porcine intestinal biological mesh in place after tumour excision.
Case 2
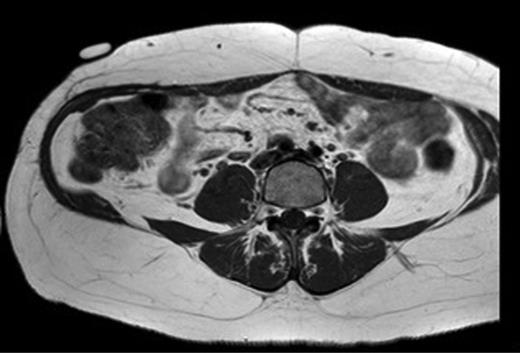
A 31-year-old man presented to his General Practitioner in May 2012 with a 6-week history of a painful mass in the right iliac fossa. There was no regional lymphadenopathy and hip examination was unremarkable. He was a smoker with a BMI of 33 and had undergone a right-sided inguinal hernia synthetic mesh repair in 2010. MRI with contrast confirmed a 6.9 × 6.7 × 4.6 cm mass invading the abdominal wall musculature (Fig. 4). Radiological features were suggestive of sarcoma or aggressive fibromatosis. Histology from an ultrasound-guided biopsy revealed aggressive fibromatosis. The patient opted for a surgical excision of the mass with abdominal wall reconstruction. The tumour was excised with the internal oblique, leaving the peritoneum and external ring cord structures intact. A 13 × 15 cm sheet of porcine intestinal biological mesh was used to repair the abdominal wall defect (Fig. 5). Inferiorly, the mesh was doubled over in the pre-peritoneal space to reconstruct the inguinal ligament, and sutured to the external oblique. The histology confirmed a final diagnosis of aggressive fibromatosis excised with 1 mm margins. At 24 months, there was good wound healing and no hernia, seroma or evidence of recurrence on follow-up MRI.
T1 MRI showing a soft tissue tumour of the anterior abdominal wall involving external oblique and internal oblique and not involving the peritoneum.
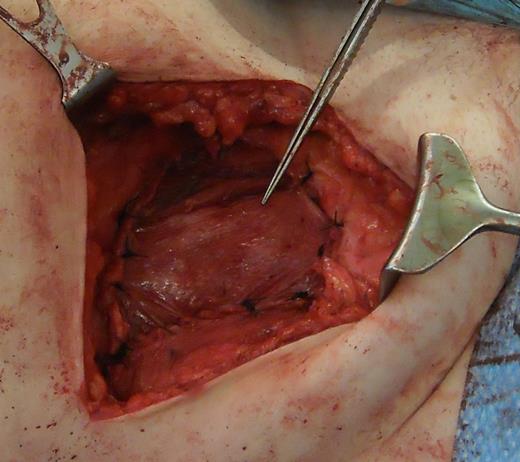
Depicting the use of porcine intestinal biological mesh to repair an anterior abdominal wall defect left by aggressive fibromatosis.
DISCUSSION
Large soft tissue tumours of the abdominal wall are very rare. When faced with selecting an appropriate technique for both of the reported procedures, consideration of the defect size (both being larger than 10 cm), the absence of any potential contamination and the excellent degree of cutaneous integrity were all central to the decision to use the porcine intestinal biological graft. A further alternative is the use of autogenous tissue in the form of advancement or regional flaps. This is considered an excellent option when the cutaneous coverage is inadequate to maintain a successful closure with mesh alone [6]. When used at the peritoneal surface, laminar prostheses have been reported as having a lower incidence of adhesion formation, which is considered to be secondary to early mesotheliation [7]. Biological tissue grafts are increasingly being utilized in abdominal wall repair. Importantly, they permit and encourage host tissue incorporation, promote neovascularization and, in theory, degrade in response to increasing wound strength and fascia formation [8]. Although superior with regard to biocompatibility, allogenic grafts (e.g. Alloderm®) used in complex abdominal wall repairs have been shown to be more prone to stretch and have up to an 80% recurrence rate when used in a bridged repair (compared with a reinforced repair) [9]. With specific regard to the two cases discussed, alternative methods to a biological mesh for reconstruction were considered; the use of a biological or synthetic graft was deemed more appropriate than a local tissue flap as both biopsy results were suggestive of aggressive soft tissue tumours. An important factor not addressed by this case report is the long-term outcome of patients who have undergone abdominal wall reconstruction with a biological mesh following tumour excision. Nevertheless, most of the reported complications relating to this option including infection, hernia and chronic inflammation are observed in the early follow-up and we have not observed these problems.
The choice of technique for abdominal wall reconstruction following excision of a large abdominal wall soft tissue tumour is a difficult clinical decision. Though biological meshes are costlier than the synthetic meshes, reduction of postoperative complications may justify the use of the biological graft. The two cases reported highlight the necessity to consider each patient according to factors such as the defect size and location, the overlying skin integrity and the sterility of the wound. The porcine intestinal biological graft thus appears a suitable alternative choice for successful repair of abdominal wall defects with good incorporation and minimal postoperative morbidity in patients with large anterior/antero-lateral abdominal wall defects following soft tissue tumour excision.
CONFLICT OF INTEREST STATEMENT
None declared.



