-
PDF
- Split View
-
Views
-
Cite
Cite
Daniela Zanotti, Mohamed Elkalaawy, Borzoueh Mohammadi, Majid Hashemi, Andrew Jenkinson, Marco Adamo, Gastro-cutaneous fistula 4 years after a fully resolved staple line leak in sleeve gastrectomy, Journal of Surgical Case Reports, Volume 2015, Issue 12, December 2015, rjv152, https://doi.org/10.1093/jscr/rjv152
Close - Share Icon Share
Abstract
Laparoscopic sleeve gastrectomy (LSG) has become a mainstream procedure in the management of obesity. Staple line leak is a challenging complication. We report a unique case of successfully treated leak after sleeve gastrectomy, presented ex novo 4 years later as a gastro-cutaneous fistula (GCF). Nothing similar was found in the literature. A 31-year-old woman underwent an LSG, complicated by an early type I leak treated successfully. After 4 years of clinical remission, the leak presented as a GCF. The conservative approach failed and a laparoscopic fistulectomy was first attempted, but after recurrence a completion gastrectomy was performed. A staple line leak is one of the most important complications after sleeve gastrectomy. Once chronic it evolves into GCF, the treatment of which is challenging. Given the absence of guidelines, experience is fundamental in its management. In our case, eventually a total gastrectomy was required.
INTRODUCTION
Obesity is a leading problem in western countries. Laparoscopic sleeve gastrectomy (LSG) has become one of the commonest bariatric procedures. One of its most feared complications is leak along the staple line, commonly occurring at the angle of His [1–3]. These leaks are known to be difficult to treat and can results in cutaneous fistula, sepsis and even death [3].
We present a particular case of leak after LSG, fully treated with laparoscopy and drainage, which presented 4 years later as a complex gastro-cutaneous fistula (GCF), eventually necessitating stomach resection.
CASE REPORT
A 31-year-old woman (115 kg, body mass index 40 kg/m2) underwent an LSG in May 2010. A sleeve was fashioned according to our standard technique under a 32-Fr orogastric bougie and stapling was commenced 5 cm from the pylorus. Intraoperative methylene blue test (MBT) was negative. Two days postoperatively, she developed abdominal pain, fever and raised inflammatory markers. The patient was re-laparoscoped and a small proximal staple line leak was found. This was primarily repaired and two 30Fr (French) Robinson drains were left. A repeated MBT 5 days later demonstrated ongoing leak. Parenteral nutrition (TPN) and antibiotic therapy were started. Two gastrograffin swallows (GS) and one MBT performed in the following weeks demonstrated a small persistent leak. A conservative management was elected at this stage and eventually inflammatory markers normalized, and antibiotics were no longer needed. Finally, no leak was demonstrated on a GS and after 63 days patient was discharged. On her last follow-up 2 years later, the patient was well and examination of her abdomen was unremarkable.
In April 2014, she presented with a 4-day history of intermittent fevers, vomiting and abdominal pain. On examination, a subcutaneous left upper quadrant swelling was found. An abdominal computed tomography (CT) demonstrated a subcutaneous collection communicating with an intra-abdominal collection extending to the gastric remnant. The patient underwent incision and drainage of the abdominal wall abscess and an oesophago-gastro-duodenoscopy (OGD), which showed a pinpoint opening 2 cm below the gastro-oesophageal junction, just above the staple line, close to the previous leak site. Findings were compatible with chronic GCF. A week later a GS could not demonstrate any leak and patient was discharged.
An outpatient OGD, with the intent to close the fistula, was carried out; the tract was defined after injection of contrast and stabilized with two clips (Fig. 1).
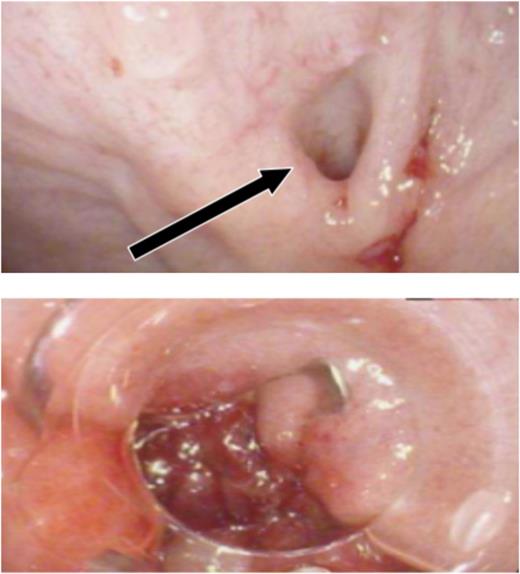
Endoscopic attempt to close the fistula. Oesophago-gastro-duodenoscopy sequence that shows the opening point of the fistula (arrow) and its stabilization with clips.
Three months later, patient presented with recurrent abdominal wall abscess. A GS demonstrated ongoing leak from the GCF (Fig. 2). On 8 September 2014, she underwent a laparoscopic fistulectomy. The fistula tract was transected and the gastric part of fistula excised. The spleen was involved in the inflammatory cavity, but splenectomy was eventually avoided. Since the gastric sleeve was healthy, it was decided not to resect the remaining stomach. No evidence of a fistula was detected on a GS performed at the 6-week postoperative check (Fig. 3).
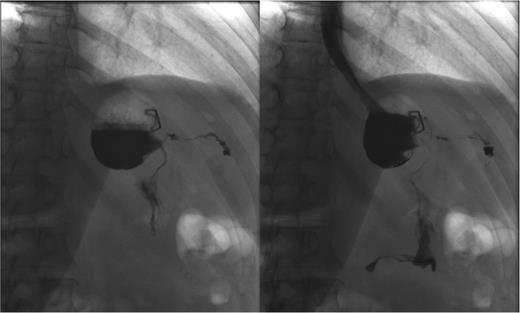
Persistent leak detected with GS. The images clearly show persistence of the leak along with the fistula tract. One of the endoscopic clips previously positioned is also visible.
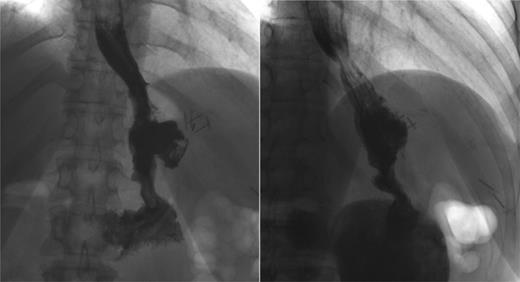
Postoperative GS. Two months after the laparoscopic fistulectomy, no persistent leak or fistula tract can be demonstrated.
In December, the patient presented with abdominal pain and a palpable left upper quadrant mass. CT showed extravasation of oral contrast from the proximal site of the gastric sleeve in communication with a sub-left diaphragmatic collection (Fig. 4).
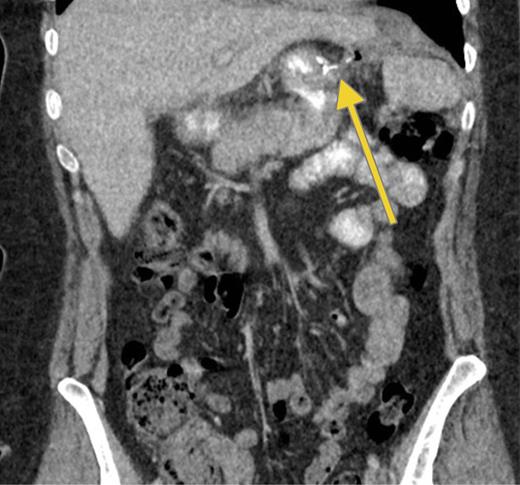
Recurrence. Computed tomography scan with oral contrast demonstrating a persistent small leak (arrow) 3 weeks after a negative GS.
After a multicentre complex benign oesophago-gastric multidisciplinary team (MDT) discussion, indication for a total gastrectomy was given. On 12 January 2015, patient underwent a laparoscopic completion of gastrectomy and Roux-en-Y oesophago-jejunostomy (Fig. 5). GS a week later showed no leak. The patient recovered well with no complication. At last follow-up, she remained well.
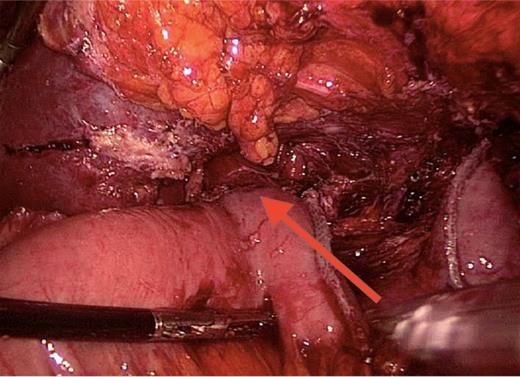
Roux-en-Y reconstruction. Laparoscopic total gastrectomy performed 5 years after the sleeve gastrectomy. Oesophago-jejunal anastomosis is indicated by the arrow.
DISCUSSION
Morbid obesity is a common disease affecting >300 million adults worldwide [1].
LSG is a relatively new option which use is rapidly growing as a primary bariatric procedure [1, 3, 4] due to remarkable outcomes. However, it can be associated with significant morbidity and leak is one of the most severe of its complications, with an incidence between 0 and 7% [5].
Based on the time of appearance, leaks have been classified as early (postoperative day, POD 1–3), intermediate (POD 4–7) and late (POD ≥8) [1, 3]. The negative pressure inside the thorax makes the angle of His their most common location.
Guidelines for the management of staple line leak have not been provided yet. A negative intraoperative MBT does not eliminate the possibility of a leak [6]. The earlier the diagnosis, the higher the chance to successfully treat the leak. Reported treatments include early oversewing, drainage and endoscopic clipping; conservative management with TPN, high-dose proton pump inhibitors and antibiotics; fibrin glue, stents, Roux-loop, and even total gastrectomy have been suggested for persistent fistulas [2–4, 6, 7].
The case we reported is unprecedented in the literature. An early leak, diagnosed by CT and promptly treated with laparoscopic surgical repair and drainage, TPN and antibiotics, presented as a GCF (after 4 years of clinical quiescence).
A GCF represents a connection between the stomach and the skin, most likely due to tissue ischaemia [6, 8] and occurs due to epithelialization of the fistula tract. The management varies according to the gravity of clinical presentation, ranging from a direct suture, through endoscopic treatment (clips or stent), to a total gastrectomy. Reported spontaneous closure rates are ∼6% [2, 4, 6, 7].
In our case, having promptly diagnosed and treated the leak, we performed regular MBT and GS to follow its course. After 4 years, symptoms recurred and a CT scan showed the presence of a GCF, suggesting the persistence of the leak during the entire time. Initially, a conservative approach was attempted, but the failure of nonoperative treatment necessitated a more aggressive surgical remedy. A laparoscopic fistulectomy was attempted first, but eventually a total gastrectomy was required.
CONFLICT OF INTEREST STATEMENT
None declared.
ACKNOWLEDGEMENTS
We owe our special acknowledgements to Prof. Muntzer Mughal, who chaired the London multicentre complex benign oesophago-gastric MDT, the input of which this work has benefited.



