-
PDF
- Split View
-
Views
-
Cite
Cite
Andrew R. Baxter, Elliot Newman, Cristina H. Hajdu, Solitary fibrous tumor of the pancreas, Journal of Surgical Case Reports, Volume 2015, Issue 12, December 2015, rjv144, https://doi.org/10.1093/jscr/rjv144
Close - Share Icon Share
Abstract
Solitary fibrous tumors (SFTs) are rare mesenchymal neoplasms of fibroblastic origin. Most commonly they affect the pleura but they been described in other viscera. SFT of the pancreas is extremely rare, and only eight cases have been reported to date. We perform a literature review and report a ninth case. The patient is a 54-year-old African-American female who presented with several months of abdominal pain. Abdominal radiography demonstrated a lesion in the head of the pancreas, and she underwent a Whipple operation. Pathology demonstrated SFT of the pancreas. She is alive and well 1 year post-operatively. SFT of the pancreas predominately affects middle-aged women. These tumors are difficult to distinguish radiologically from neuroendocrine tumors. While SFT of the pancreas tend to have an indolent course, there is the potential for malignancy. We recommend complete surgical excision.
INTRODUCTION
Solitary fibrous tumors (SFTs) constitute a group of non-epithelial tumors with spindle cell features. SFTs of the pancreas are exceptionally rare, and only eight cases have been reported to date [1–8]. These tumors present with vague abdominal symptoms or are discovered incidentally on abdominal radiography. Radiologically, they are difficult to distinguish from neuroendocrine tumors. They occur predominantly in middle-aged women and have an indolent course. Herein, we report an additional case of SFT of the pancreas and provide a review of the literature with outcomes and follow-up of cases reported so far.
CASE REPORT
The patient is a 58-year-old African-American female who presented with several months of left lower quadrant pain. A computed tomography (CT) and magnetic resource imaging (MRI) of abdomen/pelvis demonstrated a 3.8-cm hyper vascular mass in the head of the pancreas without any evidence of distant metastasis or spread to the surrounding vasculature (Figs. 1 and 2). The differential diagnosis includes endocrine tumor, gastrointestinal stromal tumor, sarcoma, solid pseudo-papillary tumor, inflammatory pseudotumour/tumor forming autoimmune pancreatitis and SFT. An endoscopic ultrasound with fine-needle aspiration was nondiagnostic. The carcinoembryonic antigen (CEA) and cancer antigen 19-9 (CA 19-9) were both mildly elevated at 6.5 (nl range 0–5.0) and 39 (nl range 0–37), respectively. Functional endocrine hormone studies were significant only for a mildly elevated gastrin level of 214, but the insulin, glucagon and vasoactive intestinal peptide (VIP) levels were all within normal limits. Her review of symptoms was negative for jaundice or weight loss. A Whipple procedure was performed. The patient had an uncomplicated hospital course and was discharged Post-op Day 7. She is alive and well 2 years post-operatively.
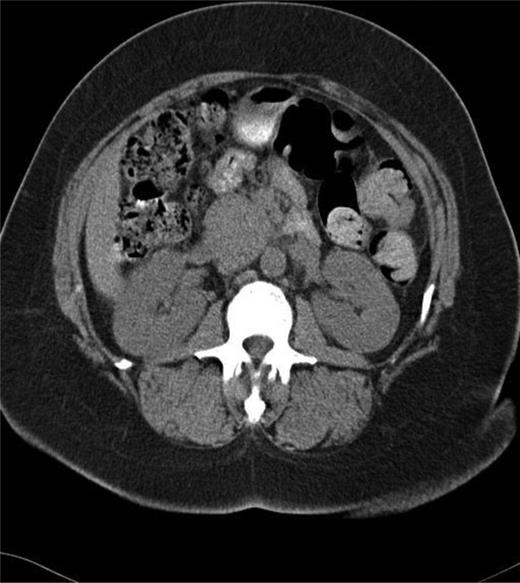
CT of the abdomen and pelvis showing mass in head of the pancreas.
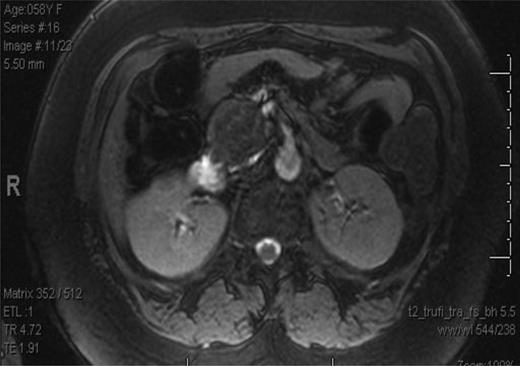
MRI of the abdomen and pelvis showing mass in head of the pancreas.
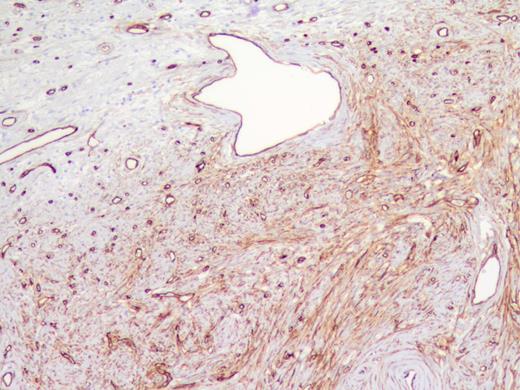
The specimen was a 3.5 ×3 ×3-cm white, firm, well-circumscribed mass located in the head of the pancreas (Fig. 3). The mass was found to be compressing the distal aspect of the main pancreatic duct. All 15 lymph nodes received in the specimen were negative. Histologically, the tumor is well circumscribed from normal pancreatic tissue and composed of spindle cells that in some areas form short ill-defined fascicles and in other areas are randomly arranged in a dense fibrohyaline stroma. A well-developed vascular network was visualized throughout the tumor with vessels of a stag horn appearance with thin hyaline wall and tumor growth around (Figs 4 and 5). No significant mitotic activity or tumor necrosis was noted. Immunostaining was positive for CD34 (Fig. 6) and BLC-2; focally for B-catenin, and focally weak staining for CD99, compatible with fibroblastic origin. The tumor cells were negative for the following cell markers: CD117, CAM5.2, AE1/AE3, EMA, synaptophysin, chromogranin, CD56, PR, SMA, Desmin, S100, MelanA and HMB45. The M1B1 proliferation index was low (<5%). These findings in combination are consistent with a SFT.
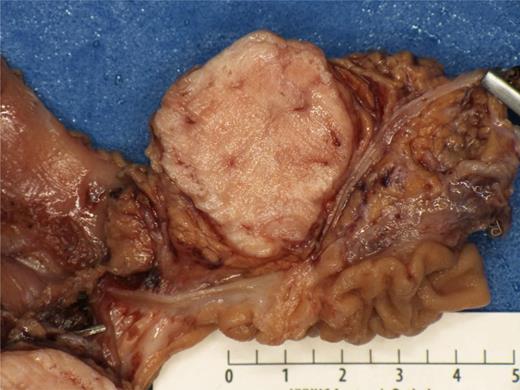
Gross pathology showing a 3.5 ×3 ×3-cm white, firm, well-circumscribed mass located in the head of the pancreas.
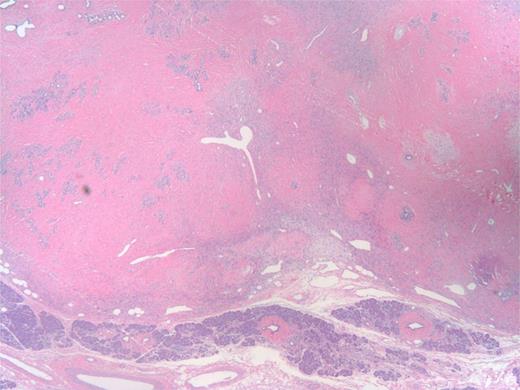
Low power of tumor (upper part) and uninvolved pancreas (bottom). The tumor has stag horn vessels, cellular areas of spindle cell proliferation and hyalinized ‘keloid areas’.
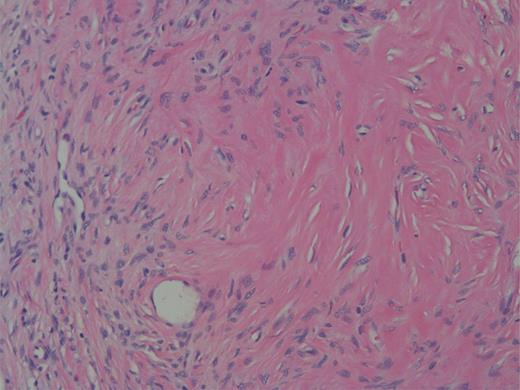
High power showing spindle cells of minimal nuclear pleomorphisms.
DISCUSSION
We performed a literature search using the following combination of keywords; Pancreas, SFT and stromal tumor. We included any case report of SFT that involved the pancreas. We then contacted all the authors of the respective case reports to obtain follow-up information. The ratio of female to male patients was 7:2, with a mean age of 56.2 (range 41–78). The mean tumor size was 5.3 cm (range 2–13 cm). One patient died on Post-operative Day 3 from surgical complications, but otherwise everyone else is alive and well with no signs of recurrence according to their latest follow-up (Table 1) [1–10].
Synopsis of the nine cases of SFT of the pancreas reported in the literature, including our own case with the reference, age, sex, type of surgery and follow-up.
| Sex . | Age . | Tumor location in the pancreas . | Tumor size . | Type of Surgery performed . | Follow-up . | Reference . |
|---|---|---|---|---|---|---|
| Female | 50 | Body | 5.5 cm | Distal pancreatectomy | Disease free 20 months post-op | Luttges et al. |
| Male | 41 | Body | 13 cm | Enucleation | Died Post-op Day 3 from surgical complications | Chatti et al. |
| Female | 41 | Neck | 2 cm | Laparoscopic enucleation | Disease free 7 months post-op | Miyamoto et al. |
| Female | 62 | Head | 3 cm | Pylorus sparing pancreoduodenctomy | Disease free 16 months post-op | Gardini et al. |
| Male | 54 | Body | 7.6 cm | Central Pancreatectomy | Disease free 70 months post-op | Kwon et al. |
| Female | 78 | Body | 5 cm | Distal pancreatectomy | Disease free 7 months post-op | Srinivasan et al. |
| Female | 67 | Uncinate process | 2.6 cm | Whipple | Alive and well 6 months post-op | Chetty et al. |
| Female | 55 | Head | 6 cm | Whipple | Alive and well 3 years post-op | Sugawara |
| Female | 58 | Head | 3.3 cm | Whipple | Alive and well 1 year post-op | Current case |
| Sex . | Age . | Tumor location in the pancreas . | Tumor size . | Type of Surgery performed . | Follow-up . | Reference . |
|---|---|---|---|---|---|---|
| Female | 50 | Body | 5.5 cm | Distal pancreatectomy | Disease free 20 months post-op | Luttges et al. |
| Male | 41 | Body | 13 cm | Enucleation | Died Post-op Day 3 from surgical complications | Chatti et al. |
| Female | 41 | Neck | 2 cm | Laparoscopic enucleation | Disease free 7 months post-op | Miyamoto et al. |
| Female | 62 | Head | 3 cm | Pylorus sparing pancreoduodenctomy | Disease free 16 months post-op | Gardini et al. |
| Male | 54 | Body | 7.6 cm | Central Pancreatectomy | Disease free 70 months post-op | Kwon et al. |
| Female | 78 | Body | 5 cm | Distal pancreatectomy | Disease free 7 months post-op | Srinivasan et al. |
| Female | 67 | Uncinate process | 2.6 cm | Whipple | Alive and well 6 months post-op | Chetty et al. |
| Female | 55 | Head | 6 cm | Whipple | Alive and well 3 years post-op | Sugawara |
| Female | 58 | Head | 3.3 cm | Whipple | Alive and well 1 year post-op | Current case |
Synopsis of the nine cases of SFT of the pancreas reported in the literature, including our own case with the reference, age, sex, type of surgery and follow-up.
| Sex . | Age . | Tumor location in the pancreas . | Tumor size . | Type of Surgery performed . | Follow-up . | Reference . |
|---|---|---|---|---|---|---|
| Female | 50 | Body | 5.5 cm | Distal pancreatectomy | Disease free 20 months post-op | Luttges et al. |
| Male | 41 | Body | 13 cm | Enucleation | Died Post-op Day 3 from surgical complications | Chatti et al. |
| Female | 41 | Neck | 2 cm | Laparoscopic enucleation | Disease free 7 months post-op | Miyamoto et al. |
| Female | 62 | Head | 3 cm | Pylorus sparing pancreoduodenctomy | Disease free 16 months post-op | Gardini et al. |
| Male | 54 | Body | 7.6 cm | Central Pancreatectomy | Disease free 70 months post-op | Kwon et al. |
| Female | 78 | Body | 5 cm | Distal pancreatectomy | Disease free 7 months post-op | Srinivasan et al. |
| Female | 67 | Uncinate process | 2.6 cm | Whipple | Alive and well 6 months post-op | Chetty et al. |
| Female | 55 | Head | 6 cm | Whipple | Alive and well 3 years post-op | Sugawara |
| Female | 58 | Head | 3.3 cm | Whipple | Alive and well 1 year post-op | Current case |
| Sex . | Age . | Tumor location in the pancreas . | Tumor size . | Type of Surgery performed . | Follow-up . | Reference . |
|---|---|---|---|---|---|---|
| Female | 50 | Body | 5.5 cm | Distal pancreatectomy | Disease free 20 months post-op | Luttges et al. |
| Male | 41 | Body | 13 cm | Enucleation | Died Post-op Day 3 from surgical complications | Chatti et al. |
| Female | 41 | Neck | 2 cm | Laparoscopic enucleation | Disease free 7 months post-op | Miyamoto et al. |
| Female | 62 | Head | 3 cm | Pylorus sparing pancreoduodenctomy | Disease free 16 months post-op | Gardini et al. |
| Male | 54 | Body | 7.6 cm | Central Pancreatectomy | Disease free 70 months post-op | Kwon et al. |
| Female | 78 | Body | 5 cm | Distal pancreatectomy | Disease free 7 months post-op | Srinivasan et al. |
| Female | 67 | Uncinate process | 2.6 cm | Whipple | Alive and well 6 months post-op | Chetty et al. |
| Female | 55 | Head | 6 cm | Whipple | Alive and well 3 years post-op | Sugawara |
| Female | 58 | Head | 3.3 cm | Whipple | Alive and well 1 year post-op | Current case |
SFTs are mesenchymal neoplasms that may be found in the pleura, soft tissue and viscera [9]. The majority of tumors have been reported in the pleura, and to date roughly 800 have been described in the literature. While these lesions appear to be predominately benign in nature, ∼12% are identified as malignant and lead to death through metastatic disease or local recurrence. The histologic characteristics associated with malignancy are high cellularity, cellular pleomorphism, greater than 4 mitotic figures per 10 high-power fields, large necrotic or hemorrhagic areas and surrounding tissue invasion [8].
SFTs of the pancreas are exceedingly rare, and to date, only eight cases have been reported in the literature [1–6, 9, 10]. The current case represents the ninth. To date, no malignant lesions have been reported, but there may be the potential for malignant disease or transformation of benign tumors into malignancy over time based on the clinical findings of SFTs found in other locations [7, 8]. Given that little is known about the natural course of this rare entity, we attempted to contact authors of these case reports to find additional follow-up information. The patient from Kwon et al. had a recent CT and was alive and well with no signs of recurrence 70 months post-op. The patient from Sugawara et al. was still alive and well 3 years post-op. The average length of follow-up was 19.3 months with no reports of recurrence thus far.
These tumors are difficult to distinguish radiologically from endocrine tumors as they appear hypervascular and well circumscribed on CT scan. The mainstay of diagnosis is histological. Particularly, the growth pattern and immunochemistry are helpful in differentiating SFTs from other mesenchymal tumors. These tumors tend to demonstrate spindle cells that grow in patternless arrangements with varying amounts of collagen and that stain positive for the cell markers CD34, CD99 and BCL-2.
Surgical excision is the recommended treatment for this rare disease entity, and although there is limited experience with SFTs of the pancreas, it appears that the prognosis is good especially if they have a favorable histological profile on generous tissue sampling and thorough microscopic evaluation. In terms of the extent of surgical resection, complete surgical resection is acceptable. Enucleation has been attempted in only two patients, and one died Post-op Day 3 from surgical complication [1, 10]. No instances of recurrences have been reported in the pancreas thus far; however, the experience is limited, and longer term follow-up is necessary.
CONFLICT OF INTEREST STATEMENT
None declared.



