-
PDF
- Split View
-
Views
-
Cite
Cite
Yosuke Matsumura, Junichi Matsumoto, Takeo Kurita, Taku Oshima, Noriyuki Hattori, Takayuki Toma, William Arthur Teeter, Shigeto Oda, Atraumatic splenic rupture cases presenting with hemorrhagic shock and coagulopathy treated by splenic artery occlusion using a microballoon catheter before splenectomy, Journal of Surgical Case Reports, Volume 2015, Issue 10, October 2015, rjv121, https://doi.org/10.1093/jscr/rjv121
Close - Share Icon Share
Abstract
Atraumatic splenic rupture (ASR) is an uncommon pathologic condition in which bleeding from the spleen occurs for a variety of nontraumatic reasons. While the current trend in traumatic splenic rupture is nonoperative management including transcatheter arterial embolization, the current recommendation for the treatment of most patients with ASR is splenectomy. In this report, we describe two cases of ASR presenting with hemorrhagic shock and complicated by anticoagulation therapy. In patients with severe hemorrhagic shock and coagulopathy, a damage control strategy is recommended. Our successful treatment of these patients included a three-step strategy as a damage control: (i) rapid transient hemostasis by splenic artery occlusion using a microballoon catheter, (ii) damage control resuscitation and (iii) splenectomy as a definitive hemostatic treatment.
INTRODUCTION
Atraumatic splenic rupture (ASR) is an uncommon pathologic condition in which bleeding from the spleen occurs for a variety of reasons [1–3]. While the current trend in traumatic splenic rupture is nonoperative management including transcatheter arterial embolization [4, 5], splenectomy is recommended in most patients with ASR as a consequence of underlying splenic pathology such as bacterial infection, malarial infection, hematological disorders, drugs or amyloidosis [1].
Two patients with ASR, concomitant hemorrhagic shock and anticoagulation therapy, presented to us for definitive treatment. Our successful treatment algorithm included a three-step strategy: (i) rapid transient hemostasis by splenic artery occlusion using a microballoon catheter, (ii) damage control resuscitation in the intensive care unit (ICU) and (iii) splenectomy as a definitive hemostatic treatment.
CASE REPORT
Case 1
A 66-year-old man presented after suffering sudden hypotension and abdominal distension, followed by a rapid and progressive anemia. After arriving at hospital, he collapsed and went into cardiac arrest. Return of spontaneous circulation occurred after 36 min of cardiopulmonary resuscitation and rapid blood transfusion. Abdominal contrast-enhanced computed tomography (CECT) revealed a large hematoma around the spleen and liver, in paracolic gutters and the pelvis with active arterial extravasation and apparent capsular disruption (Fig. 1). He was transferred to our hospital for definitive, emergency hemostasis. At admission, his systolic blood pressure (BP) was 90 mmHg; heart rate (HR), 80 bpm; hemoglobin (Hb) level, 6.7 g/dl; hematocrit (Ht), 19.0%; prothrombin time-international normalized ratio (PT-INR), 2.16 and activated partial thromboplastin time (APTT), 63.9 s (under massive transfusion). He was currently on warfarin therapy for anticoagulation following a total aortic arch replacement including aortic valve replacement at the age of 60. He presented with no history of trauma, and Moraxella catarrhalis had been detected in a blood culture taken on initial presentation. We made a diagnosis of ASR secondary to infection exacerbated by anticoagulation therapy. We first planned to perform a splenectomy as a definitive hemostatic treatment. However, his transfusion requirement was massive and the risk of significantly worsened hemorrhage during any operative procedure was increased by his iatrogenic coagulopathy. After discussion with surgery team and interventional radiology (IR) team, transient occlusion of the splenic artery using a microballoon catheter was performed (LOGOS, Piolax, Inc., Kanagawa, Japan; arrival to occlusion, 90 min; arrival to angiography suite, 60 min; procedure time to occlusion, 15 min; Fig. 2). Soon after balloon occlusion, the patient's hemodynamic state improved and his anemia stabilized. Perioperatively, 6 units of red cell concentrate (RCC) and 10 units of fresh frozen plasma (FFP) were transfused. The patient's coagulopathy improved on hospital Day 2 (Ht, 25%; PT-INR, 1.35 and APTT, 33.5 s) and was taken to the operating theater for open splenectomy. The spleen was swollen and capsular rupture was indeed identified, but there was no finding of a solid tumor or abscess formation. Bone marrow aspiration did not reveal any evidence of a hematological disorder. There was no subsequent hemorrhage postoperatively. He was extubated on Day 4, and was discharged from the ICU on Day 6. The patient was transferred to another hospital on Day 25 without residual deficits or complications from his cardiac arrest.
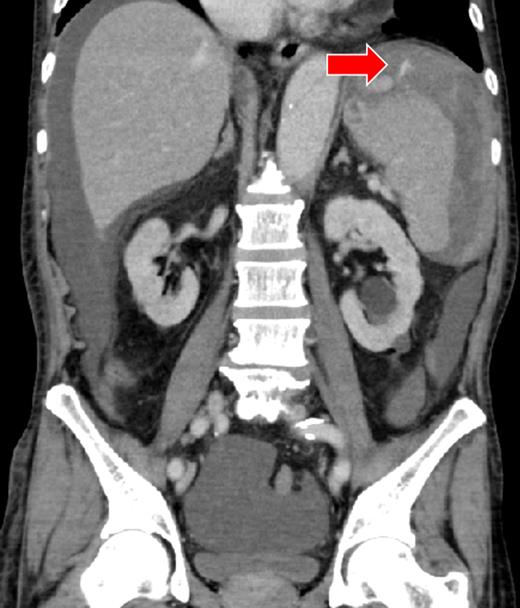
There was a massive hematoma in the peritoneum surrounding the spleen and the liver, and extending into the paracolic gutter and the pelvic cavity. The capsule of the spleen was disrupted, and active arterial extravasation can be seen around the spleen (arrow).
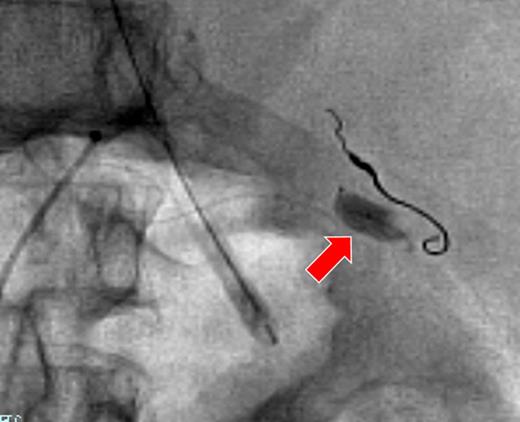
The right gastroepiploic artery with platinum coils after embolization. The microballoon catheter was placed in the splenic artery (arrow).
Case 2
A 52-year-old woman was admitted to the emergency department with altered mental status. On admission, her mental status improved to E3V4M6 on the Glasgow Coma Scale with a BP of 95/72 mmHg and HR of 92 bpm. She was found to be anemic and coagulopathic (Hb, 9.6 g/dl; Ht, 31.0%; PT-INR, 2.68 and APTT, 38.4 s). She had a history of mitral valve replacement 3 months ago and was taking warfarin and sarpogrelate. CECT showed a giant splenic artery aneurysm, splenic laceration and hematoma (Fig. 3). A diagnosis of hemorrhagic shock secondary to ruptured splenic aneurysm was made and IR was consulted. The source of hemorrhage could not be identified because of the giant aneurysm and therefore hemorrhagic shock persisted, despite aggressive resuscitation. Localization of the hemorrhagic source was abandoned and transient occlusion of the splenic artery using a microballoon catheter was performed (arrival to occlusion, 140 min; arrival to angiography suite, 110 min and procedure time to occlusion, 17 min). The hemorrhage and patient's hemodynamics subsequently improved after a total of 4 units of RCC and 6 units of FFP being transfused. A splenectomy was performed on Day 2 after correction of the patient's coagulopathy (Ht, 27%; PT-INR, 1.02 and APTT, 33.8 s). On examination, the giant splenic aneurysm was found to be a pseudoaneurysm complicated by an abscess at the hilum of the spleen, yielding a final diagnosis of ASR due to infection. The patient was moved to the general ward on Day 3, and discharged from the hospital on Day 12.
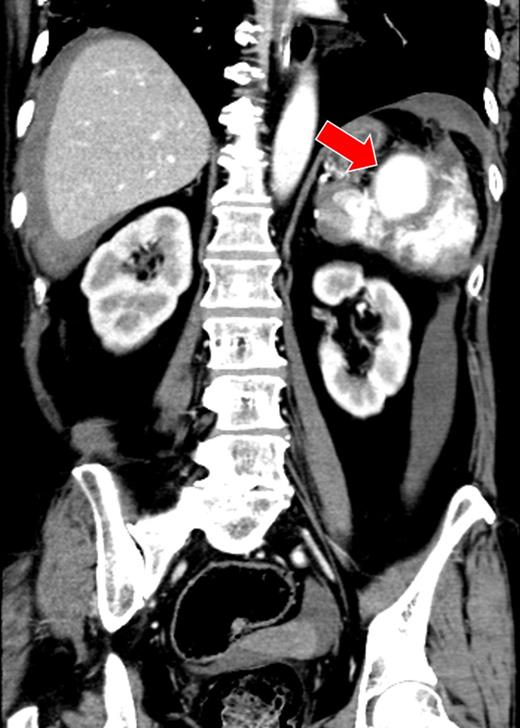
There was a massive hematoma surrounding the spleen and the liver. A giant splenic aneurysm (40 × 50 mm) and splenic laceration are seen (arrow).
DISCUSSION
Splenectomy is still considered the treatment of choice for ASR. However, IR hemostasis in emergency or trauma patients is gaining acceptance [4]. Our three-step strategy using temporary balloon occlusion was chosen over the more widely used coils, because balloon occlusion occludes the vessel lumen completely and is therefore effective in the setting of coagulopathy. Combined with critical care resuscitation and operative intervention, IR may allow a more rapid and safer hemostatic strategy than conventional IR or surgery alone. There have been few reports on the effectiveness of balloon catheters used in damage control interventional radiology for trauma or critical hemorrhage [6]. However, we believe they represent a useful hemostatic device for transient, proximal bleeding control, especially in hemorrhagic shock with coagulopathy.
We should also consider the type of balloon catheter used for transient hemostasis. Conventional balloon catheters (e.g. Selecon MP, Terumo Clinical Supply, Gifu, Japan, or Moiyan, Tokai Medical Products, Aichi, Japan) could be used as well. However, anatomical and/or technical problems might make it difficult to select and advance the catheter in similar cases. As described in the presented cases, the microballoon catheter (e.g. LOGOS, Piolax, Inc. or Attendant, Terumo Clinical Supply) could be placed in the target vessel rapidly and easily.
In conclusion, the presented cases demonstrate that splenic artery occlusion using a microballoon catheter before splenectomy can be a useful hemostatic strategy in ASR patients with hemorrhagic shock and coagulopathy.
CONFLICT OF INTEREST STATEMENT
None declared.



