-
PDF
- Split View
-
Views
-
Cite
Cite
Shareef M. Syed, Simon Moradian, Mohammed Ahmed, Umair Ahmed, Samuel Shaheen, Vasanth Stalin, A benign gastric ulcer eroding into a splenic artery pseudoaneurysm presenting as a massive upper gastrointestinal bleed, Journal of Surgical Case Reports, Volume 2014, Issue 11, November 2014, rju102, https://doi.org/10.1093/jscr/rju102
Close - Share Icon Share
Abstract
Upper gastrointestinal (UGI) bleeding secondary to a ruptured splenic artery (SA) pseudoaneurysm into the stomach is a rare but a life-threatening condition. Owing to the low prevalence, it remains a diagnostic and therapeutic challenge. A frail 77-year-old Caucasian female presented with epigastric pain and hematemesis. Endoscopy was non-diagnostic for an etiology. She then underwent diagnostic angiography that revealed an SA pseudoaneurysm with active contrast extravasation into the stomach. Subsequent transcatheter arterial coil embolization was conducted of the SA. The patient was subsequently taken for a partial gastrectomy, distal pancreatectomy and splenectomy. She had an uncomplicated postoperative course.
Diagnosis of an UGI bleeding secondary to a ruptured SA pseudoaneurysm into the stomach remains difficult. However, we report that in a hemodynamically stable patient, a multidisciplinary approach can be taken, with interval optimization of the patient prior to definitive surgery for a satisfactory outcome.
INTRODUCTION
Upper gastrointestinal bleeding is a common presentation in the USA with significant morbidity and mortality if not treated appropriately. The annual incidence of acute UGI bleeding is reported as ∼100 per 100 000, with a mortality rate reported between 2.5 and 10%. In over 75% of cases, the bleeding is secondary to non-variceal causes. In the majority of cases, ulcers, erosions and esophagitis are the major causes. Rupture of SA aneurysm or pseudoaneurysm, with visceral communication presenting as gastrointestinal bleeding is a very rare cause. There have only been relatively few cases reported in the literature. If the patients' hemodynamic status permits, preoperative diagnosis can be helpful to the surgeon in performing the optimal intervention for this condition. Standard over-sewing of a ‘bleeding gastric ulcer’ would leave the patient's underlying etiology untreated and almost certain to rebleed and needing repeat intervention [1].
We report on the case of a frail 77-year-old multiparous patient who presented with a large gastric ulcer, which had eroded posteriorly causing a pseudoaneurysm of the SA and ultimate rupture, causing several episodes of UGI bleeding. She was successfully treated using a multidisciplinary approach of endoscopy, angiography and ultimately surgery.
CASE REPORT
A frail 77-year-old multiparous Caucasian female with a history of aspirin and naproxen use, presented to an outside hospital with epigastric pain and an episode of hematemesis. She had no prior history of peptic ulcer disease, alcoholism or pancreatitis. The patient was hemodynamically stable with an acute anemia (hemoglobin of 7.7 g/dl), which prompted transfusion of 2 units of PRBC and transfer to a tertiary care facility. Upon transfer, she underwent an emergent upper endoscopy, which revealed a large amount of blood with clots in the lumen of the stomach. However, sufficient suctioning of the large intra-gastric clots was not possible; hence, an underlying etiology was not obtainable. The patient subsequently underwent a diagnostic angiography, which revealed a 4 cm mid-SA pseudoaneurysm with active contrast extravasation into the stomach which can be seen in Figs 1 and 2. The radiologist decided to perform a transcatheter arterial coil embolization of the artery to halt the active bleeding. A post procedure angiography revealed no further contrast extravasation. A planned upper endoscopy was repeated the following day that revealed some residual clotted blood in the stomach, no active bleeding and a deep 4 cm gastric ulcer. Due to the high risk of re-bleeding, the patient was optimized physiologically and consented and prepared for exploration. Intraoperatively we observed that the SA pseudoaneurysm was densely adherent to the posterior wall of the stomach, and coursing through an inflamed distal pancreas. We decided to proceed with an en bloc resection of SA pseudoaneurysm, distal pancreatectomy, partial gastrectomy and splenectomy. After the celiac axis was surgically defined, proximal and distal SA controls were obtained and a circumferential posterior gastrotomy was made. There was a visible posterior gastric mucosa to splenic arterial fistula, with the radiologically inserted embolic coils clearly visible. This can be seen in surgical specimen (Figs 3 and 4). Once the stomach was reflected, the splenic vessels and the pancreas were divided and the specimen removed. The pathology reports indicated active gastritis with transmural ulceration into the splenic artery (SA) and adjacent pancreatic tissue. No malignant cells were identified in the gastric or pancreatic tissue and the SA was not aneurysmal (Fig. 5). During the postoperative period, the patient had an uncomplicated recovery and was discharged on day 5.
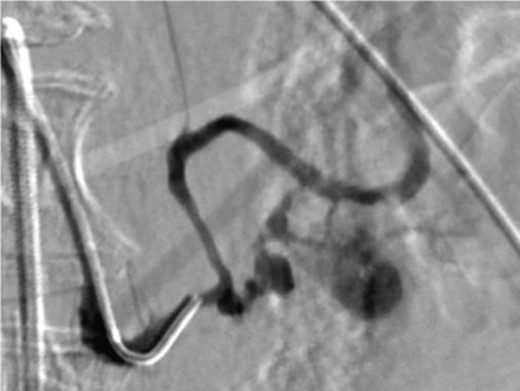
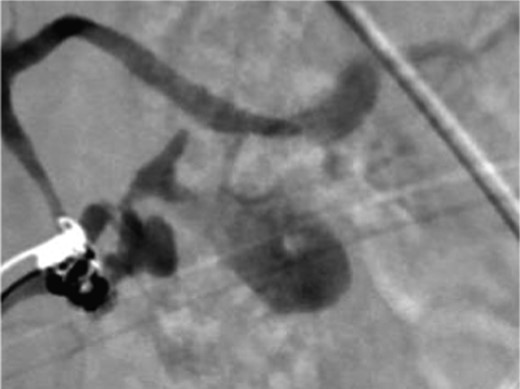
Angiography image showing splenic artery pseudoaneurysm with contrast extravasation.
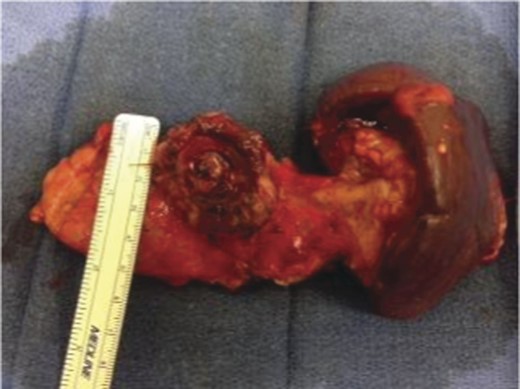
Surgical specimen of en bloc partial gastrectomy, distal pancreatectomy and splenectomy.
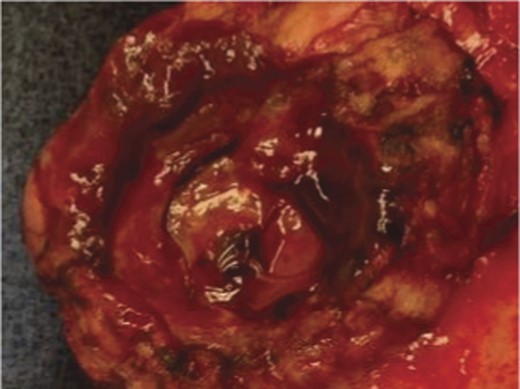
Surgical specimen of en bloc partial gastrectomy, distal pancreatectomy and splenectomy. Embolic coils visible in arterial lumen.
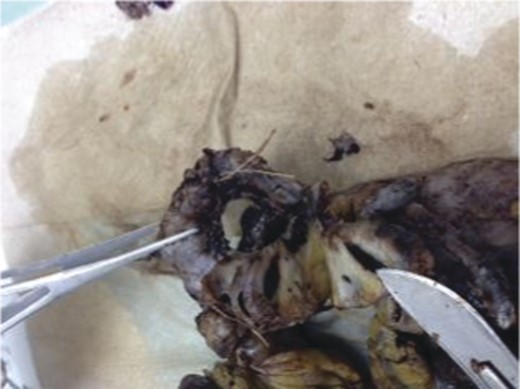
Pathologic specimen–Gastro–splenic artery fistula. Normal caliber splenic artery.
DISCUSSION
Splanchnic artery aneurysms are rare; however, the SA is the most common site [2]. Aneurysmal erosion and rupture into surrounding structures causing bleeding into the lesser sac or hemoperitoneum, upper and lower gastrointestinal bleeding via fistulizing into the stomach and colon, respectively, and bleeding into the pancreatic duct (hemosuccus pancreaticus) have been previously described [3–5]. SA aneurysms can be found in all age groups with peaks in the fifth and sixth decades. Eighty-seven percent of SA aneurysms are found in women and 80% are multiparous [5]. SA pseudoaneurysms are even rarer than true aneurysms [6]. It has been reported that pseudoaneurysms are always symptomatic unlike the majority of true aneurysms, which are usually asymptomatic [7]. However, unlike true aneurysms, the size of the SA pseudoaneurysm is not a predictor of rupture [6]. There are a number of etiologies for SA pseudoaneurysm including pancreatitis (52%), abdominal trauma (29%), postoperative complication (3%) and peptic ulcer disease (2%) [8]. The incidences of presentation are: upper or lower GI bleed (26.2%), hemosuccus pancreaticus (20.3%), hematemesis (14.8%) and with abdominal pain (29.5%) [8].
It appears that the true SA aneurysm causes pressure on surrounding viscera as it increases in size. During this time a fistulus connection is formed, and once it ruptures, it can present as GI bleeding. Hence, the aneurysm is the causative element. However, in the case of an SA pseudoaneurysm, a pathology of the surrounding viscera leads to vessel wall contact with digestive gastric or pancreatic enzymes facilitating a fistula and resultant bleeding. In our case it appeared that the large gastric ulcer had eroded posteriorly into the pancreas and SA, causing a pseudoaneurysm and subsequent rupture. The fistula was clearly evident intraoperatively, with the embolic coils plainly visible through the stomach wall.
It has been hypothesized that surgical management of ruptured aneurysms and pseudoaneurysms of the SA usually consist of splenectomy or splenopancreatectomy; however, asymptomatic true aneurysms can be treated with splenopancreatic preservation [9]. Recently TAE has become increasing utilized in the management of such bleeds. However, this may not represent adequate definitive management in a patient with an SA aneurysm or pseudoaneurysm with GI bleeding, as there is by definition a fistulus connection. It is unsurprising that there have been several reports of post procedural embolic coil migration [9]. We believe that the presence of an SA pseudoaneurysm with GI bleeding can initially be diagnosed and managed using angiography and TAE; however, definitive surgery to remove the underlying etiology must be strongly considered to reduce future morbidity in surgically acceptable candidates. If the surgical risk is prohibitive, then close endoscopic surveillance should be conducted.
GI bleeding secondary to visceral–SA pseudoaneurysm fistula is a rare. Prompt resuscitation following standard algorithms must be conducted. A high index of suspicion must be held, especially in patients with a history of pancreatitis. In the presence of hemodynamic stability, angiography can be used to diagnose this rare cause. TAE can also be used to manage any acute active bleeding if noted. However, long-term prospective outcomes of this alone have not been published, and reports of complications, including coil migration, have been observed. Hence definitive surgery must be considered to eradicate the underlying etiology and reduce further morbidity.
REFERENCES
- angiogram
- pseudoaneurysm
- hemorrhage
- epigastric pain
- embolization
- endoscopy
- gastric ulcer
- hematemesis
- extravasation of diagnostic and therapeutic materials
- frail elderly
- rupture
- splenectomy
- splenic artery
- surgical procedures, operative
- european continental ancestry group
- diagnosis
- stomach
- upper gastrointestinal bleeding
- subtotal gastrectomy
- pancreatectomy, distal
- causality



