-
PDF
- Split View
-
Views
-
Cite
Cite
Masato Kanzaki, Takuma Kikkawa, Kei Sakamoto, Hideyuki Maeda, Naoko Wachi, Hiroshi Komine, Kunihiro Oyama, Masahide Murasugi, Takamasa Onuki, Three-dimensional simulation, surgical navigation and thoracoscopic lung resection, Journal of Surgical Case Reports, Volume 2013, Issue 3, March 2013, rjt015, https://doi.org/10.1093/jscr/rjt015
Close - Share Icon Share
Abstract
This report describes a 3-dimensional (3-D) video-assisted thoracoscopic lung resection guided by a 3-D video navigation system having a patient-specific 3-D reconstructed pulmonary model obtained by preoperative simulation. A 78-year-old man was found to have a small solitary pulmonary nodule in the left upper lobe in chest computed tomography. By a virtual 3-D pulmonary model the tumor was found to be involved in two subsegments (S1 + 2c and S3a). Complete video-assisted thoracoscopic surgery bi-subsegmentectomy was selected in simulation and was performed with lymph node dissection. A 3-D digital vision system was used for 3-D thoracoscopic performance. Wearing 3-D glasses, the patient's actual reconstructed 3-D model on 3-D liquid-crystal displays was observed, and the 3-D intraoperative field and the picture of 3-D reconstructed pulmonary model were compared.
INTRODUCTION
Although conventional open thoracotomy has been replaced with video-assisted thoracoscopic surgery (VATS) for many pleuro-pulmonary diseases, video-assisted thoracoscopic lung resection such as segmentectomy and subsegmentectomy requires surgeons to have much experience and skill for performing the procedure safely. Recently, 3-dimensional (3-D) images, which help surgeons, have been used in preoperative simulation for VATS lung resection [1].
Previously, our department has reported a patient-specific virtual 3-D pulmonary model for thoracoscopic lung resections [2, 3]. This report describes 3-D video-assisted thoracoscopic lung resection guided by a 3-D video navigation system having a patient-specific 3-D reconstructed pulmonary model obtained by preoperative 3-D simulation.
CASE REPORT
A 78-year-old man with a history of pulmonary tuberculosis and gastric cancer was found to have a small solitary pulmonary nodule in the left upper lobe 4 months ago in chest computed tomography (CT) done for postoperative follow-up for gastric cancer (Fig. 1A). Routine laboratory study results, including the tumor markers carcinoembryonic antigen, sialyl Lewis X-1, and squamous cell carcinoma antigen, were within normal limits. He was preoperatively staged as N0 by positron emission tomography, high-resolution CT (HRCT) of the chest and abdomen, and the magnetic resonance imaging of brain. He was also examined by thin-sliced chest CT scan. Based on thin-sliced plain chest CT scan, based on the data of which a virtual 3-D pulmonary model was designed for him on a personal computer (PC) before operation. Patient-specific 3-D pulmonary model was created by the following methods. Homemade software, called CTTRY (Tokyo Women's Medical University, Tokyo, Japan), was used [2]. By using 120 of 1-mm thin-sliced CT scan images of the tumor and the hilum in digital imaging and communications in medicine format, CTTRY allowed us to mark pulmonary arteries, veins, bronchi and tumor in the thin-sliced CT scan images. CTTRY attempted to reconstruct an anatomical model with the help of anatomically correct images (Fig. 1B). After feeding the reconstructed model images into a PC, the pulmonary arteries, veins and bronchi of patient were traced and marked by an experienced thoracic surgeon for the anatomic structure on the images. The location and thickness of the bronchi and pulmonary vascular were rendered as various-sized cylinders. Then, based on the resulting numerical data, a 3-D image, which was converted by the surface-rendering technique, was reconstructed using software (Metasequoia, http://www.metaseq.net/) (Fig. 1C). When his virtual 3-D pulmonary model was reconstructed by CTTRY, the tumor was found to be involved in two subsegments [horizontal subsegment of apicoposterior segment (S1 + 2c) and posterior subsegment of anterior segment (S3a)] (Fig. 1E). Further, the data of the reconstructed 3-D images by Metasequoia were converted with Autodesk® 3ds Max® 2012 (Autodesk, San Rafael, CA, USA) (Fig. 1D). Finally, image software, REMO Exporter® (3D Incorporated, Kanagawa, Japan), which reproduced Autodesk data with real-time computer graphics (CG), was able to indicate with a stereoscopic vision display by 3-DCG data. On a PC, reconstructed 3-D pulmonary images were provided side by side and were output to a monitor. Surgeons were able to watch the 3-DCG of the reconstructed 3-D pulmonary model with a 3-D liquid-crystal (LC) monitor by REMO Viewer® (3D Incorporated). The reconstructed 3-D images on a 3-D LC monitor were able to be manipulated by virtual surgical procedures such as reshaping, cutting and moving (Fig. 1E). The simulation result was able to allow the subsegmentectomy of S1 + 2c and S3a to be performed with a sufficient surgical margin. A 3-D digital vision system (Shinko Optical, Tokyo, Japan) was used for 3-D thoracoscopic performance. This system included a 11-mm, 30° stereo digital scope and a 3-D data-processing unit. Wearing glasses, thoracoscopic procedures were performed by the surgeons and by means of intraoperative navigation, the patient's actual reconstructed 3-D pulmonary model was able to be watched with 3-D LC displays (Fig. 2). Complete VATS bi-subsegmentectomy was selected in preoperative simulation and was performed with intraoperative assessment of lymph nodes; hilar node dissection with mediastinal lymph node sampling was done using conventional thoracoscopic instruments (Fig. 3A and B). Final histologic examination revealed T1aN0M0 pulmonary adenocarcinoma. The patient's postoperative course was uneventful.
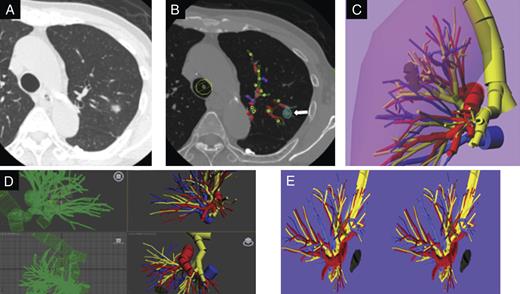
(A) Chest CT image shows a primary lesion as ground glass opacity in 2 segments (S1 + 2 and S3) of the left upper division. (B) Locations and thicknesses of tumor. Yellow dots indicate the bronchi; red dots, the pulmonary arteries; blue dots, the pulmonary veins; white, tumor, which were rendered as different-sized cylinders by the home-made software program (CTTRY). The striped rectangles of each color were a trace of the dots of each color. (C) Virtual 3-D image was reconstructed using shareware (Metasequoia). Yellow indicates the trachea and bronchi; red, pulmonry arteries; blue, pulmonary veins; the veiled area, the lung. (D) Data of the reconstructed 3-D images were converted with Autodesk® 3ds Max® 2012. (E) REMO Exporter®, which reproduces Autodesk 3ds Max data with real-time CG, can indicate with a stereoscopic vision display by 3-DCG data. On a PC, reconstructed 3-D pulmonary images were provided side-by-side and were output to a monitor. Dark yellow indicates the stapled bronchi; dark blue, resected pulmonary veins.
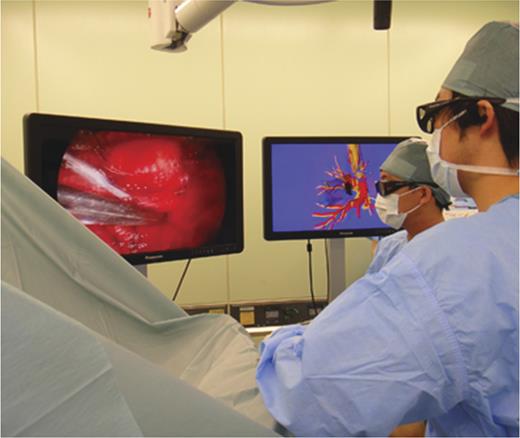
In 3-D thoracoscopic surgery with 3-D navigation, wearing glasses, comparing 3-D intraoperative field during VATS and the picture of 3-D reconstructed pulmonary model, with 3-D LC displays, the surgeons performed thoracoscopic procedures.
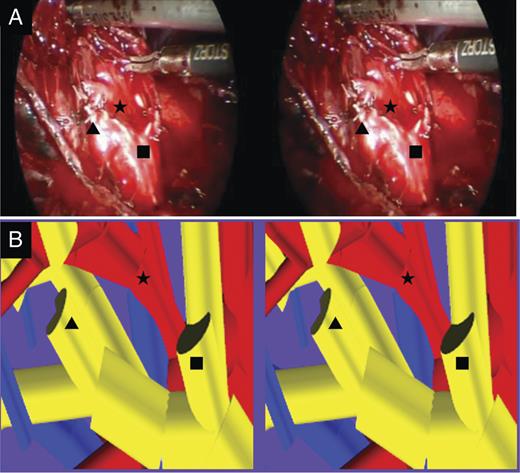
Comparison between the 3-D navigation of patient-specific 3-D reconstructed pulmonary model and the operative view. (A) The operative view of the patient was extracted from digital video data taken during the operation and was provided on a PC after operation. (B) In patient-specific 3-D image, the target bronchus and vascular branching pattern were similar to intraoperative findings. B1 + 2c (Filled Square) and B3a (Filled Triangle) were dissected and stapled separately. A1 + 2c (Filled Star) was detected.
DISCUSSION
Recently, smaller size lung cancers in a peripheral location are detected by HRCT. For these lung cancers, lung segmentectomy/subsegmentectomy has become a good option and has been reported to be able to yield promising outcomes comparable with those of lobectomy. On the other hand, video-assisted thoracoscopic lung resection such as segmentectomy and subsegmentectomy requires surgeons to have much experience and skill for performing the procedure safely.
Advances in HRCT scans have enabled reconstitution of fine virtual 3-D pulmonary models. Virtual reconstructed 3-D models can show easily the positional relationship among the target nodule, pulmonary vessels and bronchi, and allow surgeons to access more peripheral vascular branches and target segmental/subsegmenatal bronchi with an accurate detection during a thoracoscopic procedure [1]. As a result, reconstructed 3D pulmonary models guide surgeons in avoiding vascular injury in complete VATS lung resections. Further, simulation using a patient-specific 3-D pulmonary model helps surgeons to select the most suitable type of VATS lung resection [2–4].
In 3-D thoracoscopic surgery with 3-D navigation onto a 3-D LC display, depth perception is improved, compared with a conventional thoracoscopic surgery, and 3-D surgery is similar to an operative field vision of open thoracotomy [5]. Although 3-D vision was hampered by technical and financial limitations previously, a 3-D thoracoscopic system has been improved to real-time transmission. There is no study that performs 3-D endoscopic surgery using both pre-surgical 3-D simulation and actual navigation with a reconstructed patient pulmonary model. In our 3-D thoracoscopic surgery under 3-D navigation, comparing 3-D intraoperative field and the picture of 3-D reconstructed pulmonary model, surgeons simply wore glasses and were able to perform thoracoscopic surgery, such as segmentectomy and subsegementectomy, more easily and precisely. This technique can be applied to tumor <2 cm. We ensured that the safety surgical margin was >2 cm from the tumor.



