-
PDF
- Split View
-
Views
-
Cite
Cite
Settanan Plangsiri, Pat Ngamdachakij, Manisara Jirapornsuwan, Panupol Thepyasuwan, Chanakarn Sribangpleenoi, Panjapon Kitgrongpaibul, Varinthip Thongchai, Songyos Tangmesang, Simultaneous laparoscopic management of dual primary malignancies: case reports and literature review of synchronous renal cell carcinoma and colon carcinoma of sigmoid, Journal of Surgical Case Reports, Volume 2025, Issue 9, September 2025, rjaf722, https://doi.org/10.1093/jscr/rjaf722
Close - Share Icon Share
Abstract
Dual primary malignancy is a rare but possible malignancy presentation. Here, we present two cases of synchronous renal cell cancer and colon cancer with simultaneous laparoscopic management. The first patient, a 65-year-old female, reported painless hematochezia, while the second, a 73-year-old female, complained of painful bloating and constipation, along with a palpable mass in the left lower quadrant of the abdomen. A computed tomography (CT) scan of both patients’ abdomens revealed the expected colonic masses. However, surprisingly, the scans also showed hypervascular masses with peripheral enhancement in the kidneys, suggestive of renal cell carcinoma. No evidence of metastasis was found in either patient. A simultaneous laparoscopic sigmoidectomy combined with radical nephrectomy was successfully performed for both patients. Postoperative CT scans and sigmoidoscopy confirmed complete tumor resection with no evidence of local recurrence. The present case report may serve as a valuable reference for managing similar clinical scenarios in the future.
Introduction
Dual malignancy is a rare phenomenon in which two primary malignancies arise simultaneously in a single patient. This entity differs from metastasis, where a primary cancer spreads to other organs. Synchronous primary cancers in different organs present unique clinical challenges, as both must be treated concurrently.
Renal cell carcinoma (RCC) is the most common type of kidney cancer in adults, accounting for over 90% of all kidney cancer cases [1]. Multiple subtypes of RCC can be identified using histopathological and molecular techniques. Despite advances in its detection and treatment, RCC has a mortality rate of approximately 30%, the highest among all genitourinary neoplasms [2]. However, early detection and surgical removal of the cancerous lesion can result in complete remission [3].
Colorectal cancer (CRC), on the other hand, ranks fourth in cancer-related mortality worldwide, with over 900 000 deaths occurring annually [4]. The risk of developing CRC remains relatively low until the age of 45, after which it rises substantially. CRC usually starts by transforming the endothelial cell into an adenoma and, eventually, adenocarcinoma [5]. Early detection of CRC enables treatment through surgical removal of the tumor and associated lymph nodes if metastasis to the nodes is present [6, 7].
This report presents two cases of dual malignancies involving RCC and sigmoid colon carcinoma, highlighting the clinical features, diagnostic challenges, and management strategies through successful simultaneous laparoscopic resection of both tumors.
Case series
History, physical examination, and investigation
Case 1
A 65-year-old female patient presented with hematochezia for 7 months. She denied any history of abdominal pain, flank pain, genitourinary symptoms, or previous surgeries. The symptoms started with mild rectal bleeding and progressed to full hematochezia with blood clots coming out along with feces. Abdominal examination revealed a soft, nondistended abdomen without palpable masses, rebound tenderness, or guarding. The patient underwent both esophagogastroduodenoscopy (EGD) and colonoscopy. The EGD result was unremarkable. However, colonoscopy revealed a large sessile mass in the sigmoid colon, located approximately 20–25 cm from the anal verge.
Computed tomography (CT) scan of the abdomen revealed a 5.2 mm polypoid lesion at the rectosigmoid colon without evidence of pericolonic fat extension, adjacent organ invasion, or regional/distant lymph node enlargement. Incidentally, a 5 cm hypervascular mass was noted at the lower pole of the right kidney, demonstrating peripheral enhancement with central low density. CT scan of the chest revealed no lymph node enlargement or pulmonary metastasis. On CT scan, the rectosigmoid colon mass exhibited radiologic characteristics suggestive of sigmoid colon cancer, while the lower pole right kidney mass demonstrated features consistent with RCC (Fig. 1).
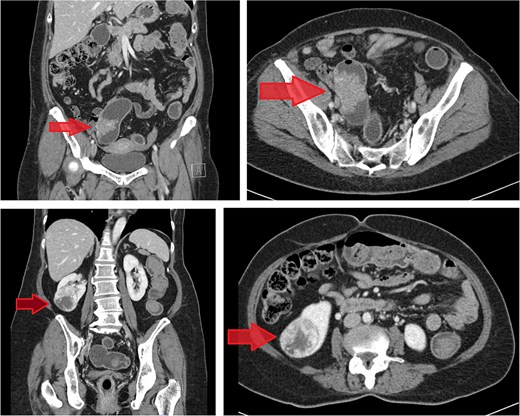
Contrast-enhanced CT images of case 1. Top-left: Coronal view of the sigmoid colon cancer. Top-right: Transverse view of the CRC. Bottom-left: Coronal view of the renal cell carcinoma (RCC). Bottom-right: Transverse view of the RCC.
Case 2
A 73-year-old female patient presents with severe abdominal pain, which is exacerbated when moving. The patient also complains of bloating and chronic constipation for the past 2 years. She had no history of previous surgery. The patient denied a history of decreasing appetite, weight loss, nausea, vomiting, hematochezia, and mucus in stool. One week before the visit to the clinic, the patient felt a mass in her left lower quadrant of the abdomen as well. The patient was sent for EGD and colonoscopy. The EGD result revealed chronic gastritis. The colonoscopy paints a different picture with a 100% circumferential proliferative mass at 25–30 cm from the anal verge and a 0.8 cm polyp at 25 cm from the anal verge (Fig. 2). Biopsy of the mass and polypectomy were done. The result revealed the mass as adenocarcinoma and the polyp as tubular adenoma without high-grade dysplasia.
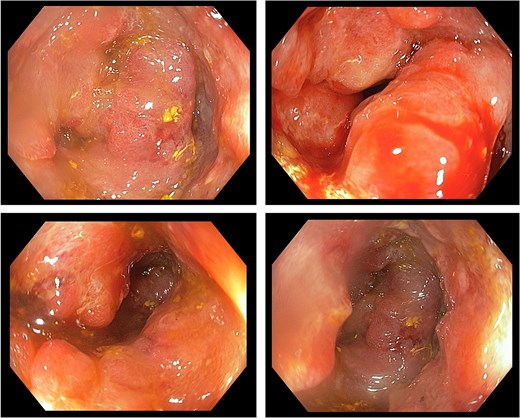
Colonoscopic image of case 2 showing a circumferential, proliferative mass occupying 100% of the lumen, located 25–30 cm from the anal verge.
CT scan of the abdomen revealed a short segment of irregular, circumferential wall thickening at the distal descending colon, measuring 1.8 cm in thickness and extending 4.8 cm in length. Moreover, it was associated with an adjacent soft tissue lesion and perilesional fat stranding. Chest CT imaging demonstrated the absence of lymph node enlargement and pulmonary metastatic lesions. Incidentally, a 6.6 cm heterogeneous, hypervascular mass was detected in the interpolar region of the right kidney, abutting the anterior renal fascia (Fig. 3).
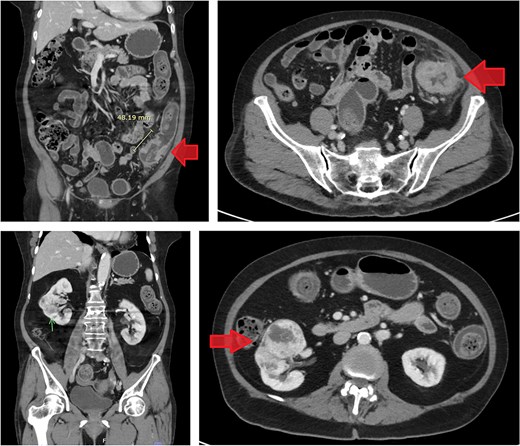
Contrast-enhanced CT images of case 2. Top-left: Coronal view of the sigmoid colon cancer. Top-right: Transverse view of the CRC. Bottom-left: Coronal view of the RCC. Bottom-right: Transverse view of the RCC.
Similar to the previous case, the imaging findings were suggestive of synchronous dual primary malignancies involving RCC and sigmoid colon cancer. Both cases were co-managed by urologists and general surgeons. Magnetic resonance imaging was not conducted in either case, as the findings from abdominal and chest CT scans, along with EGD and colonoscopy, provided sufficient evidence to support the clinical diagnosis and guide management.
Surgical procedure
In both cases, simultaneous laparoscopic surgery was performed on the patients, starting with right radical nephrectomy. The robotic approach was not chosen due to its unavailability at the author’s institution. Following dissection from the surrounding tissues, the kidney was enclosed in a retrieval bag and left in situ for concurrent removal with the colonic specimen. Subsequently, the general surgeon proceeded with a laparoscopic sigmoidectomy.
In case 1, the estimated blood loss was 160 ml. No blood transfusion was required. The procedure was completed without any intraoperative complications. The total time taken for the urologic laparoscopic excision of the right kidney was 1 h and 20 min. The total time taken for laparoscopic resection of the colon mass was 3 h and 50 min. In case 2, the estimated blood loss was 70 ml; no blood transfusion was administered, and no intraoperative complications occurred. The nephrectomy procedure took a total of 2 h to complete. The total time taken for laparoscopic sigmoidectomy was a total of 4 h and 25 min. Postoperative complications were unremarkable, and the patients were discharged after an uneventful recovery with stable vital signs and appropriate wound healing.
Operative and histopathological findings
Case 1
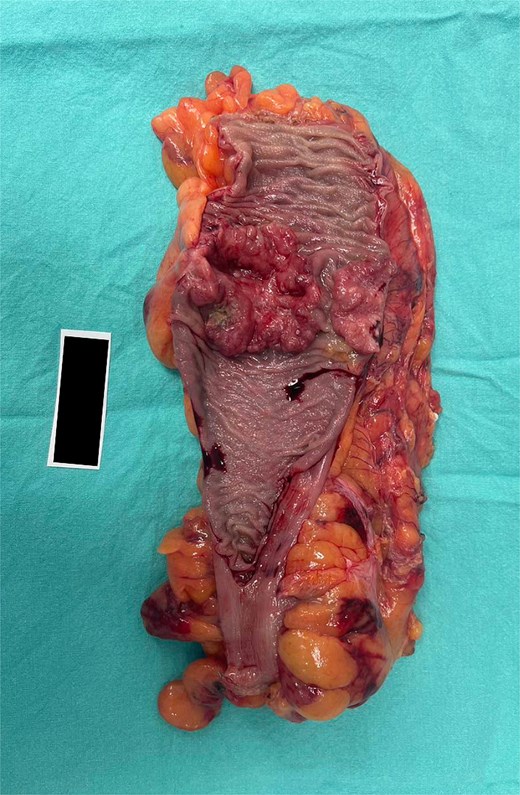
A kidney mass was found at the lower pole of the right kidney without invasion of Gerota’s fascia. A 100% circumferential fungating mass was found in the sigmoid colon without serosal invasion (Fig. 4).
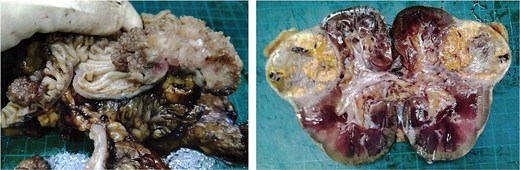
Surgical specimen of case 1 showing synchronous tumors: Sigmoid colon cancer on the right and RCC on the left.
Histopathological specimens of the kidney revealed clear cell RCC (ccRCC) (Fig. 5), measuring 4.0 × 4.0 × 3.8 cm with a histologic grade of G2. The tumor growth was limited to the kidney, with all margins negative for invasive carcinoma. Tumor necrosis was present at 10%. Lymphovascular invasion was present. This case of RCC was staged as T1aN0M0 according to the TNM classification [8]. The sigmoid colon mass analysis revealed low-budding, well-differentiated adenocarcinoma, size 6.5 cm, with submucosal invasion (T1). There is no lymphovascular or perineural invasion. The margin was negative for both proximal and distal mesenteric margins. The remaining tissue examination revealed a tubular adenoma with focal high-grade dysplasia. There were negative results for metastatic carcinoma in all 12 lymph nodes from the specimen. In this case, the TNM staging for the sigmoid colon carcinoma was determined to be T1N0M0 [9].
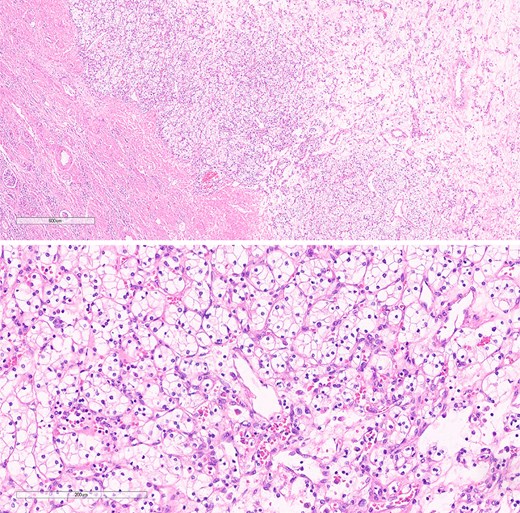
The histological section of RCC shows clear cytoplasm and cellular morphology consistent with clear cell RCC.
Case 2
The mass in the kidney turned out to be at the midpole of the right kidney. The colon mass turned out to be an 80% circumferential sigmoid mass with a proximal margin of 15 cm and a distal margin of 10 cm. There were also several IMA nodes ranging from 0.5 to 1 cm (Fig. 6).
Surgical specimen from case 2 demonstrating sigmoid colon cancer.
The pathological analysis of the kidney mass was revealed to be ccRCC, measuring 6.0 × 4.7 × 3.5 cm with a histologic grade of G2. The tumor extension was limited to the lower pole of the right kidney. All margins were negative for invasive carcinoma, and 10% tumor necrosis was identified. In this case, the TNM staging of RCC was classified as T1bN0M0 [8]. The sigmoid colon mass was revealed to be grade G2—moderately differentiated, adenocarcinoma measuring 5 cm at the maximal diameter (Fig. 7). The cancer has shown invasion through the muscularis propria into the pericolic fatty tissue. Fortunately, the proximal resection, distal resection, and mesenteric resection margins showed no involvement by the invasive carcinoma. In addition, all of the 15 biopsied lymph nodes showed no metastasis. In this case, the sigmoid colon cancer was staged as T2N0M0 [9].
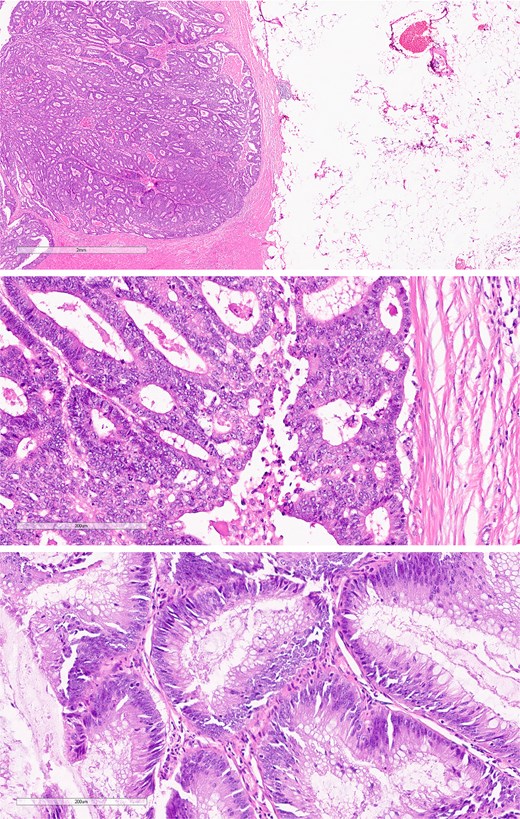
Histological section of sigmoid colon cancer showing atypical glandular structures consistent with adenocarcinoma.
Further treatment and follow-up
Both patients underwent complete surgical resection. Follow-up CT scan of the whole abdomen, performed 3 months postoperatively, confirmed complete tumor removal from both the kidney and sigmoid colon without evidence of recurrence or metastasis. Laboratory values for each patient pre- and postoperatively are given below:
Case 1: Preoperatively, the serum creatinine was 0.71 mg/dl and the estimated glomerular filtration rate (eGFR) was 90.0 ml/min. Three months after the operation, the serum creatinine increased to 1.07 mg/dl, with a corresponding eGFR of 54.8 ml/min. The carcinoembryonic antigen (CEA) level remained stable, with pre- and postoperative values reported as <1.73 ng/ml.
Case 2: Preoperatively, the serum creatinine was 0.72 mg/dl and the eGFR was 83.1 ml/min. At the 3-month postoperative follow-up, the serum creatinine was 0.94 mg/dl, and the eGFR was 59.8 ml/min. The CEA level decreased from a pre-operative value of 4.1 ng/ml to 2.3 ng/ml postoperatively.
Sigmoidoscopy revealed a patent anastomosis with no evidence of recurrent gross tumor in both patients. The patients could return to normal activities without complications.
Discussion
Synchronous RCC and CRC, though rare, have been well documented in various case reports, with reported incidence rates ranging from 0.03% to 4.85% [10]. Notably, patients with RCC appear to have a higher risk of developing subsequent primary malignancies [11]. Since CRC is more prevalent than RCC in the general population, most reported synchronous RCC and CRC cases have been detected incidentally during staging evaluations, typically via CT imaging for CRC [12], as observed in our presented cases. Additionally, the occurrence of RCC and CRC has been associated with an increased risk of developing a third malignancy, possibly due to genetic factors [13]. Therefore, careful surveillance is essential to ensure that any new malignancies are detected promptly.
In both cases, simultaneous laparoscopic surgery was performed to treat RCC and CRC, offering potential advantages such as shorter hospital stays and improved cost-effectiveness. However, a combined surgical approach may carry an increased risk of perioperative complications. In our cases, laparoscopic radical nephrectomy was selected over partial nephrectomy due to greater technical feasibility.
A key clinical challenge lies in distinguishing between metastatic disease and two primary malignancies, as this determination highly influences management strategies. Isolated metastasis of RCC to the colon is exceedingly rare, with the liver being the most common metastatic site due to the tumor’s spread via lymphatic and venous pathways [14]. Similarly, metastasis of CRC to the kidney is also considered exceptionally uncommon [15]. Therefore, careful evaluation is essential to differentiate between these possibilities, as true dual primary malignancies without metastasis require entirely different treatment approaches compared to metastatic disease. While primary tumors may be managed surgically with curative intent, metastatic disease is typically associated with a poorer prognosis and often necessitates systemic chemotherapy—unless the metastases are resectable or pose an immediate clinical concern requiring surgical intervention [16]. Physicians should also be aware that initial symptoms may be atypical and imaging findings can be ambiguous, potentially delaying diagnosis. Even in the absence of definitive radiologic evidence, prompt investigation based on clinical suspicion is essential to avoid delays in treatment [17, 18]. Surgeons should remain vigilant for the possibility of abdominal tumorous masses, which may include combinations such as ccRCC and CRC, as in our case, or other reported primary combinations, such as ccRCC and upper tract urothelial carcinoma [19].
Dual primary malignancies involving RCC and CRC are rare but possible and should not be overlooked. This case report highlights the successful diagnosis and management of such a condition through simultaneous laparoscopic nephrectomy and colon resection, performed collaboratively by general surgeons and urologists. The present case report may serve as a valuable reference for managing similar clinical scenarios in the future.
Acknowledgements
The authors thank Pokket Sirisreetreerux and Chumpon Wilasrusmee for continuous guidance throughout the writing process.
Conflict of interest statement
None declared.
Funding
None declared.
Data availability
All data was available.
Ethical statement
The study was approved by the institutional ethics committee of COA No. MURA2024/835. Written informed consent was obtained from the patients for the publication of this case report and accompanying images.



