-
PDF
- Split View
-
Views
-
Cite
Cite
Jerin Thomas, Peter Giannaris, Kelly Lee, Juan Luis Gomez Marti, Tristan Tham, Aron Z Pollack, William H Westra, Elana Opher, Charles C L Tong, Synchronous yet distinct HPV-associated sinonasal carcinomas on an immune-dysfunctional background, Journal of Surgical Case Reports, Volume 2025, Issue 9, September 2025, rjaf693, https://doi.org/10.1093/jscr/rjaf693
Close - Share Icon Share
Abstract
Human papillomavirus (HPV)-associated multiphenotypic sinonasal carcinoma (HMSC) is a relatively new classification of head and neck carcinomas that displays histological combinations of multiple different neoplasms. Despite their high-grade appearance, the disease course is often indolent. Here, we report a unique case of HMSC in which a patient with a prior history of sarcoidosis presented with two histologically, and anatomically distinct tumors in the sinonasal tract. One of the tumors was denoted as HMSC, and the other resembled a nonkeratinizing squamous cell carcinoma. Importantly, these two tumors were both found to be driven by the same high-risk HPV strain, HPV45, which has not been reported previously in HMSC. In this patient, it is important to note that the concomitant diagnosis of malignancy and sarcoidosis makes disease monitoring challenging, given the ability of sarcoidotic nodules to mimic the metabolic characteristics of tumors on PET scans.
Introduction
HMSC is a malignancy unique to the sinonasal tract [1], a mix of squamous cell carcinoma (SCC) combined with a poorly differentiated tumor with features reminiscent of salivary gland carcinomas [2]. Paradoxically, the disease course is most often indolent, with very few cases reporting death [3, 4] or metastasis [1, 3–5]. As a rare, newly classified sinonasal malignancy, the factors associated with developing this disease remain unclear, except for one report including occupational status [6]. We aim to add to the literature by describing two synchronous tumors in opposite regions of the sinonasal tract driven by HPV45, with differing histological classification in a patient with sarcoidosis.
Case report
A middle-aged patient presented with progressive right nasal airway obstruction. The patient had a history of sarcoidosis, managed with fluticasone/salmeterol. CT scan revealed an expansile soft tissue mass causing complete blockage of the right nasal passage (Fig. 1). Functional endoscopic sinus surgery (FESS) was performed to remove the mass. The mass was well localized to the lower two-thirds of the middle turbinate. It was entirely separated from the nasal floor and nasal septum. A subtotal middle turbinectomy and complete FESS were performed to obtain lateral margins. Intraoperatively, a smaller second irregular focus of tissue was noted in the left nasal passage in the left middle turbinate (Fig. 1), suggesting another tumor, and was removed during a second procedure.
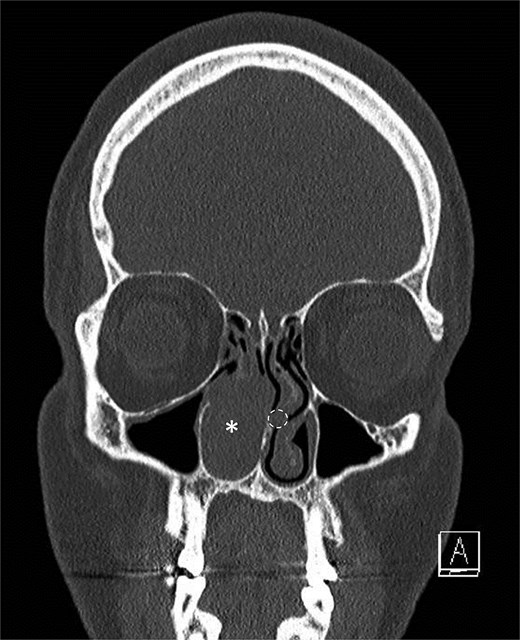
Initial scan demonstrating a mass. Coronal CT scan displaying expansile lesion in the right nasal passage denoted by asterisk. Lesion was noted to have erosion of distal nasolacrimal duct and inferior turbinate without bony erosion. Location of lesion in left nasal passage denoted by dotted circle.
Pathology from the right sided mass revealed a 5-cm tumor. Histologic examination showed focal areas resembling solid adenoid cystic carcinoma, which included microcystic spaces and eosinophilic basement membrane-like material. Most of the tumor was basaloid, composed of solid nests of small blue cells with necrosis. Scant ductal structures with focal keratinization were also present. On immunohistochemistry (IHC), the basaloid component was diffusely positive for CK7, rimmed peripherally by a single layer of p63- and p40-positive myoepithelial cells. The basaloid nests stained strongly and diffusely for p16, and ki-67 was elevated (>80%). CD117 staining was patchy and nonspecific. Lastly, the ciliated surface epithelium was replaced by SCC in situ with pagetoid spread. Epithelial markers stained in the usual fashion (full-thickness epithelium positive for p40, p63, p16, and high ki-67). Tumor cells expressed human papillomavirus (HPV) E6/E7 mRNA, confirming their HPV-related nature (Fig. 2). Ancillary studies, including INI-1 IHC and MYB::NF1B fluorescence in situ hybridization, were negative, ruling out the SMARCB1 (INI-1)-deficient sinonasal carcinoma and adenoid cystic carcinoma. Morphologic and immunohistochemical findings were diagnostic of HMSC.
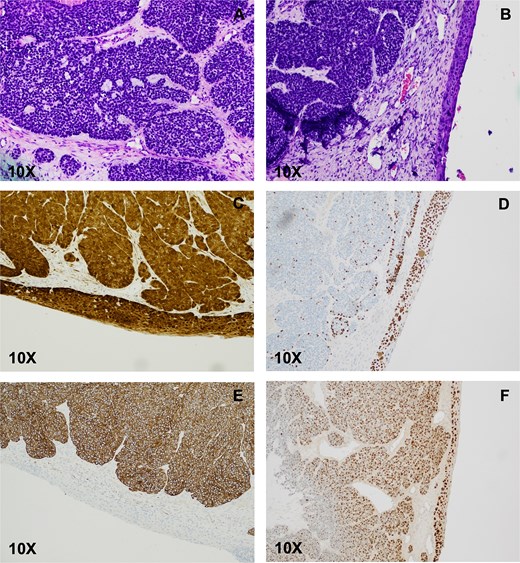
HPV-associated multiphenotypic sinonasal carcinoma. (A) Histologic examination of the right nasal cavity lesion showed a biphasic cell population composed of cells with scant cytoplasm and hyperchromatic nuclei surrounding ductal-type cells arranged in varying sizes of encapsulated solid nests with occasional microcribiform architecture. The ciliated surface epithelium showed replacement with SCC in situ (B), which stained positive for p16 (C), and p40 (D). CK-7 highlighted the basaloid nests (E). Transcriptionally active HPV was confirmed by E6/E7 positivity (F).
Histologic examination of the smaller left turbinate lesion showed SCC with papillary and in situ features. Tumor cells stained diffusely for p16, p40, and p63. Additionally, the HPV nature of the SCC was confirmed by HPV E6/E7 in situ hybridization (ISH). Likewise, CK7 was positive, and ki-67 was high (>80%). No invasive carcinoma was seen at this site. The background stroma was replaced by non-necrotizing granulomas, consistent with sarcoidosis. Both left and right turbinate neoplasms were genotyped, and surprisingly the results showed that both were HPV45-driven, making this the first reported case of HMSC caused by HPV45.
Although postoperative MRI of the neck revealed no abnormalities, skull-to-thigh 18F-fluorodeoxyglucose (18F-FDG) PET scans revealed hypermetabolic lymphadenopathy above and below the diaphragm, as well as hypermetabolic pulmonary nodules. Consultation with pulmonology suggested the findings were more consistent with stage II sarcoidosis than metastasis, but endobronchial ultrasound guided transbronchial needle aspiration (EBUS-TBNA) confirmed sarcoidosis. However, due to concerns of potential lymphovascular invasion from the left lesion, adjuvant radiotherapy was recommended by radiation oncology to reduce recurrence and/or spread. The patient underwent radiotherapy of the nasal cavity and is being followed regularly.
Discussion
HMSC is a rare tumor which, to date, has only been described in the sinonasal region and was recently incorporated into the WHO classification [7]. Apart from nonkeratinizing squamous cell carcinomas (nkSCCs) and rare, high-grade adenocarcinomas, no other association has been found between carcinomas of the sinonasal tract and transcriptionally active HPV [7]. In Western countries, the incidence of HPV-associated oropharyngeal cancer has continued to rise since the 1970s and it has now surpassed cervical cancer as the most common HPV-related cancer in the USA [8]. Similarly, incidence of HPV detection in the sinonasal tract has risen at an annual rate of 3.41% [9].
As shown in our case, HMSC is an aggressive-appearing neoplasm that displays features of salivary gland carcinoma with biphasic differentiation (ductal and myoepithelial), as well as features of SCC. The current literature reinforces the notion that it is primarily a disease with a moderate risk of local recurrence [1], but a low rate of distant metastasis [1, 3, 5] and disease-associated death [3–5]. The salivary gland component usually demonstrates a biphasic tumor composed of basaloid myoepithelial-type cells with scant cytoplasm; ductal-type cells form solid nests, with varying degrees of microcribiform architecture. High-risk HPV, most frequently HPV33, is the causative organism of HMSC [10]. Our case is unique in that a previously undescribed HPV type, HPV45, was causative of two geographically and phenotypically distinct tumors.
Sarcoidosis is a multisystem inflammatory disease characterized by noncaseating granulomas in the lymph nodes, classically presenting as bilateral hilar adenopathy that is incidentally found on chest imaging [11]. It has been well documented that immune dysfunction and chronic inflammation can contribute to oncologic pathogenesis. In sarcoidosis, some studies suggest a link to malignancy [12, 13], although no clear mechanistic link has been established. Two meta-analyses aiming to understand the relationship between sarcoidosis and cancer came to the conclusion that there is an associated risk of developing cancer [14, 15]. However, Ungprasert et al. [15] noted limitations and biases that weaken their claim. Further work is needed to clarify whether a link exists between sarcoidosis and malignancy, as well as the mechanism underlying this risk. Importantly, clinical management is complicated by the propensity of inflammatory nodules to demonstrate increased 18F-FDG uptake in a PET scan, a feature that is shared with cancer, and is evident in our own patient.
As a subclassification of sinonasal carcinomas, HMSC is still in its infancy, and further investigation is required to better understand its clinicopathologic features. We report a case of HMSC occurring alongside an anatomically separate sinonasal nkSCC, both of which were driven by HPV45.
Conflict of interest statement
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical approval
Ethical approval to report this case was obtained from the Northwell Health Institutional Review Board (#23-0823).
Informed consent
Written informed consent for publication was obtained from the patient.
Data availability
Not applicable.



