-
PDF
- Split View
-
Views
-
Cite
Cite
Yohei Kawatani, Takahisa Hirano, Takaki Hori, Inguinal hernia repair after ilio-iliac artery bypass, Journal of Surgical Case Reports, Volume 2025, Issue 8, August 2025, rjaf597, https://doi.org/10.1093/jscr/rjaf597
Close - Share Icon Share
Abstract
Inguinal hernia after lower limb artery bypass surgery using the external iliac artery is particularly rare with no standard operation method. An 81-year-old man with a history of ilio-iliac artery bypass was diagnosed with right inguinal hernia. Given that no dissection was necessary in the preperitoneal space around the external iliac artery, which was anastomosed with an artificial vessel graft, and that the procedures could be performed above the transverse abdominis, the Lichtenstein method was selected for the case. This intervention demonstrated technical challenges in creating the space for mesh placement under the artificial vessel graft. During the procedure, the artificial vessel graft and posterior wall of the inguinal canal required careful dissection due to the adhesion of these structures. The case outcome was favorable.
Introduction
In surgical treatment for iliac artery occlusion, the external iliac artery is sometimes selected for artificial arterial anastomosis. The operative region is similar to that of both ilio-iliac artery bypass and inguinal hernia repair. However, only a few reports have described the surgical repair of inguinal hernia after iliac artery bypass [1, 2], and due to its rarity, no standard method exists for this intervention. This case report presents a successful inguinal hernia treatment after an ilio-iliac artery bypass.
Case report
An 81-year-old man with a history of ilio-iliac artery bypass (Fig. 1) and left stent graft leg occlusion presented with swelling in the right lower abdomen. A diagnosis of right inguinal hernia was established (Fig. 2), and the patient was scheduled for surgical repair (Figs 3 and 4). Computed tomography revealed that the artificial vessel passed through the subcutaneous route, in which the artificial graft was anastomosed to the right external iliac artery in the preperitoneal space, and the subcutaneous tunnel (Fig. 1). Ultrasound imaging in the standing position revealed that the hernial sac originated lateral to the external iliac artery, hypogastric artery, and the point of origin of the artificial graft.
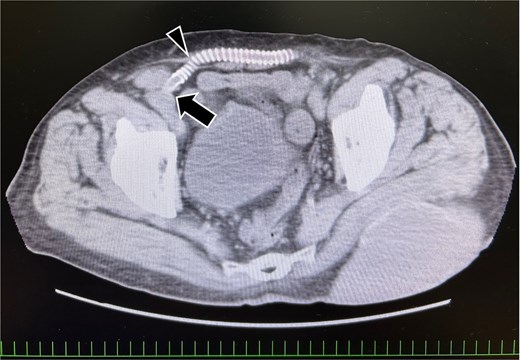
Preoperative computed tomography image showing the vessel graft on the right side. The external iliac artery and artificial vessel graft are anastomosed in the preperitoneal space (arrow). The graft running subcutaneously after originating from the abdominal wall (arrowhead).
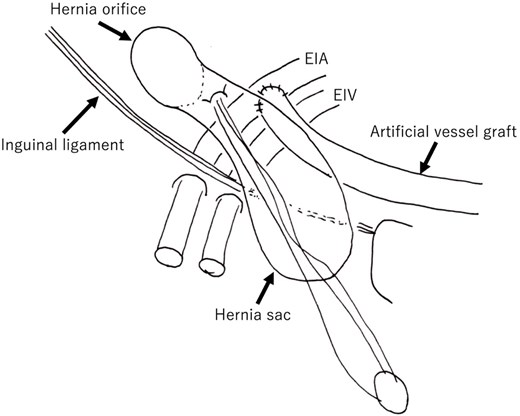
Schematic overview of the case. The orifice is lateral to the artificial vessel graft. The weakness of the posterior wall of the hernial canal was the etiology in this case. The hernial sac is overlaid onto an artificial vessel graft. EIA: External iliac artery; EIV: External iliac vein.
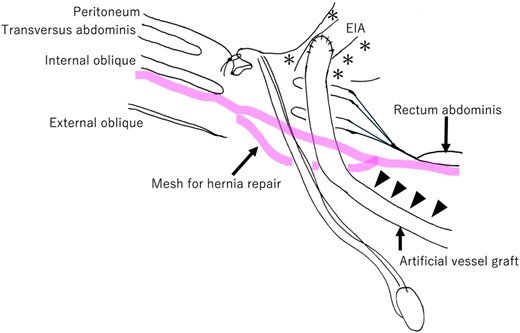
Schematic overview after hernia repair mesh placement. The artificial vessel graft and surrounding tissues were highly adherent. The artificial vessel graft is dissected from the transverse abdominis (arrowhead). The hernia repair mesh is placed between the internal oblique and external oblique muscles over the weak posterior wall of the inguinal canal. In this case, the previous surgical site around both the external iliac artery and artificial vessel graft in the preperitoneal space (*) is left untouched. EIA: External iliac artery.
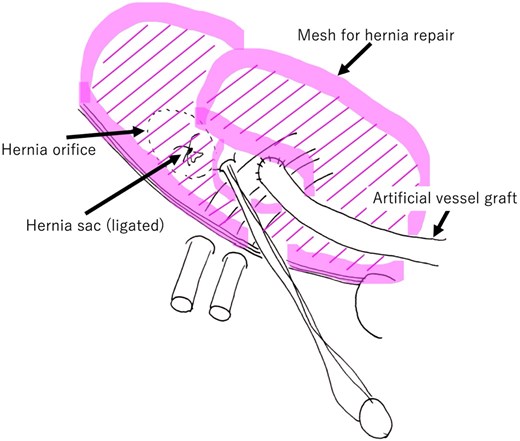
Schematic overview after hernia repair mesh placement. The mesh is placed between the internal oblique muscle and external oblique fascia over the area of the inguinal canal posterior wall.
A standard anterior-approach Lichtenstein method was performed. A 5 cm-long ordinal incision was made in the right lower abdomen after confirming the anatomical structure (Fig. 5). Subsequently, the anterior wall of the inguinal canal was opened, and both the artificial vessel graft and spermatic cord were dissected (Fig. 6). The inguinal hernia orifice was located lateral to the artificial vessel graft. However, the posterior wall of the inguinal canal was deficient. After high ligation of the hernial sac, the space under the artificial vessel was dissected to make room for hernia repair mesh placement. The artificial graft was adherent to the internal oblique fascia, and these structures were carefully dissected (Fig. 3, arrowhead).
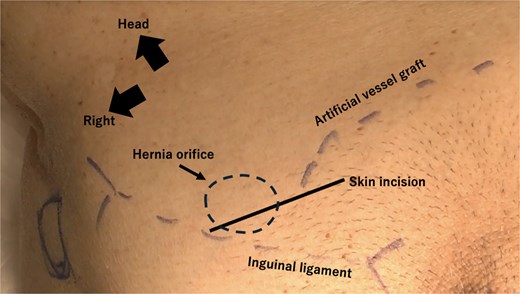
Artificial vessel graft, hernial orifice, and skin incision positions. A skin incision is made over the hernial orifice and the origin of the artificial graft, as confirmed by preoperative ultrasound. The incision is made nearly at the traditional position.
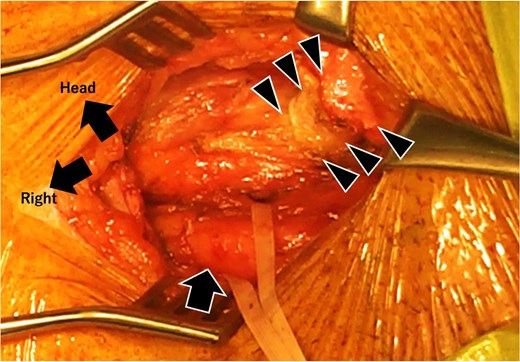
Intraoperative image after artificial vessel graft and spermatic cord dissection. The orifice of the spermatic cord (taped) is lateral to the origin of the artificial vessel graft. The posterior wall of the inguinal canal, located lateral to the artificial vessel graft, is weak and is assumed to be the etiology.
A hernia repair mesh (ProGrip™, Covidien, Mansfield, MA, USA) was placed over the internal oblique muscle (Figs 3 and 4). Around the takeoff point of the artificial graft, testicular vessels, and deferent canal, the mesh was fixed using 3–0 polypropylene sutures.
The patient was discharged without complications. No recurrence was detected at the 1-month postoperative follow-up.
Discussion
Although inguinal hernia after prostatectomy is well documented [3], its etiology remains unknown. In such cases, hernia is typically of the indirect type [4]. Conversely, inguinal hernia following iliac artery bypass grafting is rare. In the present case, the etiology was assumed to be abdominal wall weakness caused by the patient’s old age. Previous surgery was not associated with indirect hernia development in the patient. The abdominal wall medial to the external iliac artery was stiff and hard due to the adhesions related to artificial arterial graft implantation. This indicates that inguinal hernia after ilio-iliac artery bypass assumably occurs more frequently on the lateral abdominal wall than on the medial side, suggesting that indirect inguinal hernias are more frequent. Notably, one reported case [2] and the presented case involved indirect inguinal hernia.
Standard treatment approaches for inguinal hernias include the Lichtenstein method, onlay mesh repair, mesh plug occlusion, and laparoscopic repair. However, the standard treatment option for inguinal hernia after ilio-iliac bypass has not yet been established.
In this case, we selected the Lichtenstein method primarily because this approach does not require dissection in the preperitoneal space, and surgery could be performed in the space anterior to the posterior wall of the inguinal duct. In the previous surgery, the preperitoneal space was dissected for anastomosis of external iliac artery and artificial vessel graft. Adhesions resulting from these surgical procedures could complicate operative procedures, particularly dissecting interventions around the external iliac artery and artificial grafts in the preperitoneal space during preparing the site for mesh placement. The Lichtenstein method allowed us to avoid these procedures, which are necessary in laparoscopic repair. Two technical difficulties were associated with the implanted artificial vessel graft. First, the artificial vessel graft was adherent to the inguinal canal, requiring careful dissection of the vessel graft, inguinal cord, and internal oblique muscle. Second, passing the mesh through the narrow space under the artificial graft was technically challenging, as the mesh tended to adhere to the internal oblique muscle tissue (Fig. 3, arrowhead). After placement, the mesh was stable in the orifice of the artificial graft, testicular vessels, and deferent canal (Fig. 4).
Mesh plug occlusion of the hernial orifice could be proposed as another treatment option [5], given that it is a relatively easy procedure that does not require dissection in the preperitoneal space around the external iliac artery. However, we did not opt for this method as the etiology of the present case involved abdominal wall weakness based on the intraoperative findings. In this situation, the mesh reinforcement effect of the Lichtenstein method was assumed to be more preferable. Laparoscopic inguinal hernia repair has been widely used due to its favorable outcomes and reported success after femoro-femoral artery bypass surgery for leg ischemia [2]. However, this method would not be the best option after artery bypass surgery using the iliac arteries as in the case of these bypass surgeries, the preperitoneal space is dissected and an artificial graft is implanted into the space, as described above. The laparoscopic transperitoneal approach for hernia repair carries a potential risk of arterial injury in the case of dissection around the iliac artery [6] due to the nature of laparoscopic dissection procedures in that area. Adhesions increase the risk after an iliac artery bypass. In fact, Tsukuda et al. reported laparoscopic repair of indirect inguinal hernia after iliofemoral artery bypass, which could not be accomplished due to an adhesion around the artificial graft and iliac artery [1].
Conclusions
The anterior mesh reinforcement technique, referred to as the Lichtenstein method, was successfully performed to treat indirect hernia after ilio-iliac artery bypass. The case presented here demonstrates that this method is safe and effective in similar circumstances.
Author contributions
YK wrote the manuscript. All authors contributed to patient treatment. All authors reviewed and approved the final manuscript and accept responsibility for its publication.
Conflict of interest statement
The authors declare no competing interests.
Funding
No external funding was obtained for this case study.
Data availability
The data supporting the conclusions of this article are included within the article.
Ethics approval and consent to participate
Local ethical committee approved this study. The patient approved to the participation.
Consent for publication
Written consent for publication was obtained from the patient.



