-
PDF
- Split View
-
Views
-
Cite
Cite
Rawan Alhalabi, Ahmad Alsweed, Osama Hamud, Sridhar M Ramaiah, Chetan Gupta, Ubaid Shah, Dalia Belsha, Hussein Muad, Afsheen Umer, Muhammad Eyad Ba’ath, Successful treatment of giant multiple enteroatmospheric fistulas in an extremely premature infant using staged procedures. A case report and literature review, Journal of Surgical Case Reports, Volume 2025, Issue 8, August 2025, rjaf582, https://doi.org/10.1093/jscr/rjaf582
Close - Share Icon Share
Abstract
Necrotizing enterocolitis (NEC) remains a leading cause of morbidity in extremely low birth weight neonates. We report a complex surgical management of a 560 g preterm infant with NEC complicated by intestinal perforation, enteroatmospheric fistula, and prolonged open abdomen. Management included negative pressure wound therapy combined with stoma bag application, prolonged total parenteral nutrition, and delayed reconstruction using multiple anastomoses and tube stomas. Final abdominal closure was achieved with absorbable mesh and skin flaps. Despite prolonged hospitalization and multiple complications, enteral autonomy was ultimately achieved. This case highlights the challenges of managing NEC-related enteroatmospheric fistulas in extremely low birth weight infants and supports the use of negative pressure wound therapy and staged reconstruction to preserve bowel length and avoid short gut syndrome.
Introduction
Necrotizing enterocolitis (NEC) is the most common critical condition leading to surgical morbidity and mortality in extremely low birth weight (ELBW) neonates weighing less than 1000 g [1]. It is characterized by inflammation of the intestine leading to potential complications such as bowel perforation [1, 2]. Initial management often involves conservative measures [1, 3, 4].
Surgical treatment of NEC is indicated in cases of intestinal perforation, which may present with pneumoperitoneum on imaging, or in the presence of necrotic bowel segments [1, 5]. Procedures such as laparotomy are fundamental for managing these complications, with techniques such as bowel resection, primary anastomosis, or stoma formation usually implemented [2, 5]. Severe cases can result in challenging outcomes such as enteroatmospheric fistula (EAF) formation following laparostomy, which can substantially complicate postoperative recovery [4, 5].
This report highlights the surgical management and outcomes in a complicated case of NEC in an extremely premature neonate, focusing on the complexities associated with perforation, laparostomy, and fistula development.
Case report
An extremely premature surviving twin baby, born at 23 weeks of gestation, was referred to our tertiary neonatal unit for advanced care. The baby weighed 560 g. He was intubated and ventilated immediately, followed by an uneventful umbilical arterial catheter and umbilical venous catheter insertion. Trophic feeds were started on Day 4 of life. Formula feeding started on Day 7. The baby passed meconium on Day 8 and abdominal distention was noted on the same day. On Day 10, the baby developed raised inflammatory markers, hyponatremia, and acidosis. The feeding was stopped, and antibiotics were started. On Day 11, he developed septic shock. Supine and lateral decubitus abdominal X-ray was done, which showed free air, confirming intestinal perforation (Fig. 1).
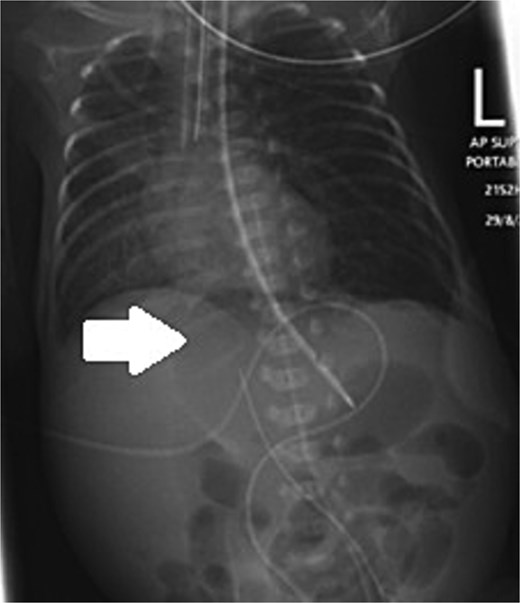
Supine and lateral decubitus abdominal X-rays on Day 11 of life showing free air (arrow), confirming intestinal perforation.
A Penrose drain was then inserted in the right lower quadrant of the abdomen for the management of perforation. A small amount of air and meconium were retrieved. Blood culture confirmed the growth of Enterococcus. The drain produced serous discharge for two more days and it was then shortened gradually and eventually removed on Day 15 of life.
On Day 17, abdominal examination was benign, and the general condition of the baby was stable; therefore, feeding was recommenced and increased gradually. The baby tolerated up to 120 ml/kg feeds and had multiple changing stools. On Day 25, the baby demonstrated abdominal distension and increased inflammatory markers. Feeding was stopped, and antibiotics restarted. The baby, however, continued to deteriorate, with increasing abdominal distension, and started to have bilious vomiting with no stools for 4 days. An ultrasound scan showed no collection, an echogenic liver, and minimal free fluid in the left side of the abdomen. An abdominal X-ray showed a relatively gasless abdomen, concerning for necrotic bowel (Fig. 2). Therefore, laparotomy was performed on Day 29.
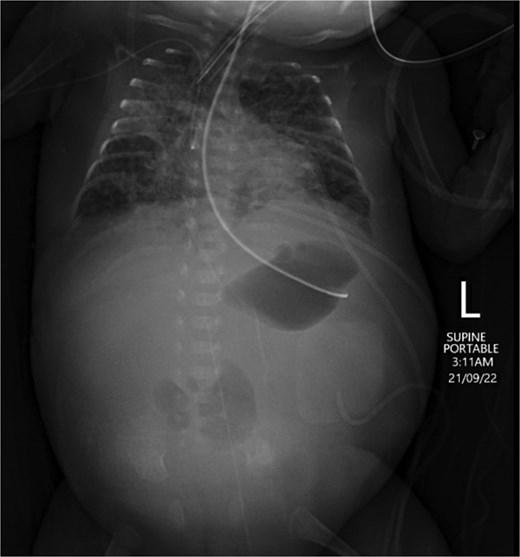
Abdominal X-ray on Day 25 demonstrating a gasless abdomen concerning for necrotic bowel.
The findings were of an enormous and fragile liver, extensive inflammatory adhesions, thick, curd-like milk-filling, fragile bowel loops, which perforated and bled profusely to the slightest touch. The bowel was so brittle it was seen to perforate spontaneously during the procedure without even handling.
After trying to establish anatomy for nearly 45–50 minutes, it was clear that proceeding further would lead to more bowel injury. The procedure was complicated by a liver laceration and some denuded bowel. The baby was starting to get hypothermic. Therefore, a proximal stoma was pulled out from the left end of the transverse laparotomy wound, around 7 cm from the duodenojejunal flexure. The abdomen was closed with four tension sutures, with a vessel loop left underneath them and pulled from either side of the wound as a drain. Hemostasis was secured, and no immediate effect on ventilation was noted.
Subsequently, plenty of serous leak from the abdomen was noted, which progressed into thin green liquid from a hole in the bowel proximal to the stoma. On Day 39 of life, he demonstrated compartment syndrome of the abdomen. Part of the abdominal wall became necrotic, and ventilation worsened. This necessitated removal of the tension sutures and the creation of a laparostomy, leaving the bowel exposed. This was managed with a nonadhesive dressing initially. Later, more and more bowel loops fistulized to the surface, ultimately coalescing into a giant EAF. This was managed by applying a stoma bag over the most proximal leaking point and then applying a negative pressure dressing over the remainder of the exposed bowel. The baby was maintained on total parenteral nutrition (TPN) besides this dressing strategy, for nearly 7 months. Gradual feeding was restarted at 2.5 months of age, reaching 12 ml 3 hourly mixed feeding through an OG tube at 6 months of age with 64 ml daily stoma output. Figure 3 shows the wound progression and the dressing applied.
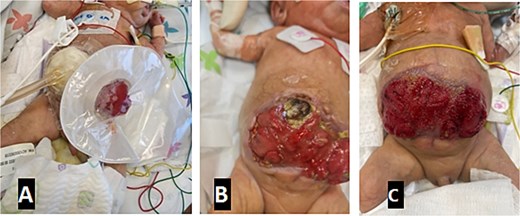
Progression of laparostomy wound and application of NPWT. Images show the evolution from initial bowel exposure to the development of a giant enteroatmospheric fistula, managed with a stoma bag and NPWT. (A) VAC + stoma bag dressing applied. (B) Four weeks post-op with a necrotic abdominal wall. (C) Wound appearance 6 months post-op.
Afterward, the patient needed two Broviac lines and multiple central lines. Weight gain was sluggish, and the child had relatively high calorie requirements, likely due to the large open wound. The infant stayed ventilator-dependent. He had multiple septic episodes and DIC, complicated by multiple cardiac arrests leading to severe neurological insults. Despite that, the family insisted on full management. The child developed mild parenteral nutrition–associated liver disease.
The infant then underwent a 12-hour laparotomy at 7 months of age when he reached 2.5 kg (around 6 months after the EAF formation). The large plate of the enteroatmospheric fistula (EAF) was gradually reconstructed into an enteric tube. This required eight anastomoses and closure of six side perforations. The resultant small intestine length was 50 cm, with preserved ileocecal valve and whole colon. Using 12 French Foley catheters, two tube ostomies were created and pulled out through the lower fascial leaflet: one proximal (7 cm from duodenojejunal flexure) and one distal. The ostomies were externalized through separate incisions in the abdominal wall.
The large fascial gap was too large to be closed primarily; it was therefore bridged with a Vicryl mesh, which was then covered with skin flaps raised superiorly and inferiorly. Figure 4 shows the appearance of the abdomen in the immediate period pre- and post-op. A distal loopogram was performed 3 weeks following the surgery to confirm the integrity and patency of the reconstruction.
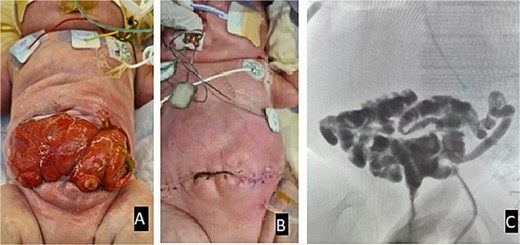
(A) Appearance of the abdomen in the immediate period pre-op. (B) Appearance of the abdomen post-op. (C) Distal loopogram shows adequate bowel length, confirming no fistula or stricture with contrast reaching the rectum.
Subsequently, the baby was gradually commenced on enteral feed through orogastric tube OGT. The enteric output was recycled between the two tube ostomies. Weight gain improved but stayed suboptimal. The child needed parenteral nutrition intermittently.
Nearly 3 months later, another surgeon attempted closure of the jejunostomy through a limited incision around the tube stomas. This procedure failed. The new wound burst and the anastomosis fistulized to the surface as an enterocutaneous fistula (ECF). Another spontaneous ECF also evolved later, as shown in Fig. 5.
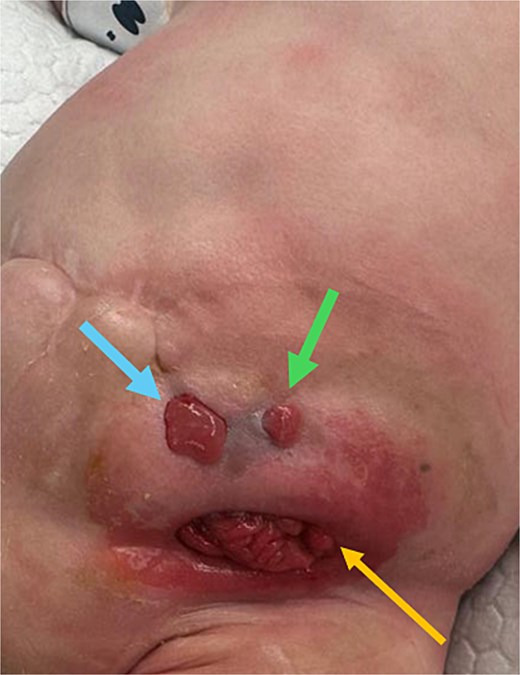
Development of new enterocutaneous fistulas after failed jejunostomy closure. Green arrow: ECF number 1. Blue arrow: ECF number 2. Yellow arrow: the new burst wound anastomosis site.
An abdominal CT scan with oral contrast revealed two ECFs: the first connected to the small bowel (Fig. 6, green arrow, corresponding to fistula number 1 in Fig. 5), and the second connected to the proximal sigmoid colon (Fig. 6, blue arrow, corresponding to fistula number 2 in Fig. 5). Furthermore, an enteroenteric fistula between the ileum and rectosigmoidal junction was noted (Fig. 7, red arrow).
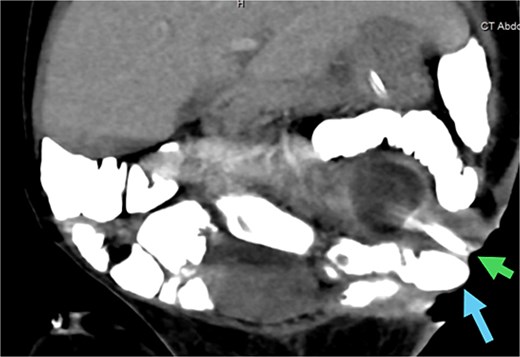
CT abdomen with oral contrast showing two enterocutaneous fistulas. Green arrow: fistula connected to the small bowel. Blue arrow: fistula connected to the proximal sigmoid colon.
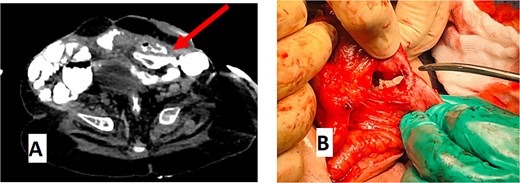
(A) CT abdomen with oral contrast showing enteroenteric fistula. The red arrow indicates a fistula between the ileum and the rectosigmoid junction. (B) Gross appearance of the enteroenteric fistula.
Two months later, a third surgeon closed the fistulas using a vertical incision, avoiding previous incisions. This was completed successfully. Direct fascial closure was possible at this stage. The final abdominal scar is shown in Fig. 8. The TPN was stopped at 20 months of age.
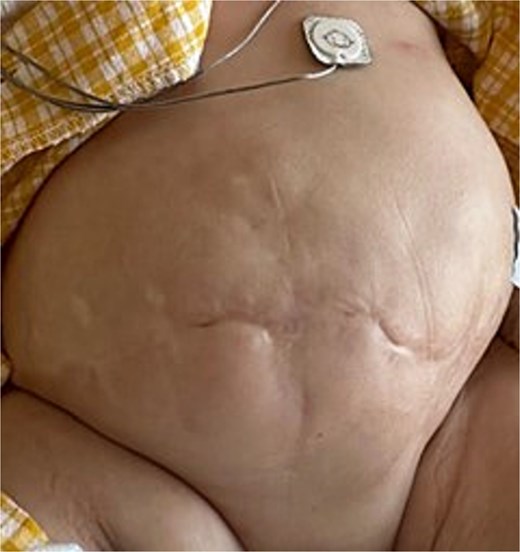
Final abdominal appearance after successful surgical closure. Direct fascial closure achieved through vertical incision; the child is shown postoperatively with intact skin coverage and healed surgical wound.
Currently, the child’s age is 2.5 years. He is receiving a ketogenic diet through an OGT. His current weight is 8.7 kg, with a Z score of −3.75. The child maintains enteral autonomy despite significant prematurity-related morbidities, such as being ventilator-dependent with a tracheostomy and chronic lung disease.
This report was meant to focus on the surgical aspects. Nonetheless, the child went through multiple complications, e.g. acute kidney injury, hyperglycemia that needed insulin infusion, neonatal jaundice, meningitis, and intracranial hemorrhage.
The child now has tracheostomy, cerebral atrophy, a fatty liver, chronic lung disease of prematurity, metabolic bone disease of prematurity, retinopathy of prematurity, exposure keratopathy, developmental delay, and seizures. The child was never discharged home.
Discussion
NEC frequently affects premature neonates in the first 10 days of life [1, 2]. Additionally, NEC has been reported to be recurrent and occurs in full-term infants as a postoperative complication [6, 7]. Severe cases are commonly complicated with perforation, peritonitis, sepsis, and mortality [1]. One-third of NEC patients require surgical intervention, but the optimal surgical management remains controversial [8]. Peritoneal drainage (PD) has been suggested as initial management for perforated NEC in ELBW infants who are too unstable for a laparotomy or as definitive management [8–10].
Whether primary laparotomy or PD is superior for treating perforation related to NEC in premature neonates is a well-recognized controversy in the surgical management of NEC. A study performed by Dimmitt et al. in 2000 showed that all patients with ELBW and perforated NEC who had a worsening clinical picture post-PD necessitating laparotomy died. The authors concluded that patients did not benefit from salvage laparotomy after the drainage [10]. Another paper was published by Eicher et al. in 2012. Six ELBW neonates with perforated intestines were treated initially with PD; two died immediately after the procedure, four needed secondary laparotomies, and one ended up with developmental delay. The author suggested early laparotomy rather than PD should be performed in such infants with bowel perforation. This approach was associated with less morbidity and mortality [8]. A recent observational study was completed by Geng et al. in 2018. The study revealed that premature patients who undergo PD for NEC suffer a higher mortality rate, sustain a longer Neonatal Intensive Care Unit (NICU) stay, and have extended dependence on TPN [9]. On the other hand, a meta-analysis accomplished in 2022 showed no significant difference in mortality between PD and laparotomy as the initial surgical intervention [11]. Although PD was associated with lower survival rates in observational studies, a systematic review and meta-analysis showed PD as the primary surgical treatment for NEC has similar survival rates when analyzing data from randomized controlled trials only. This suggests that the apparent worse results of PD in observational studies might be due to selection bias [12].
Chyme reinfusion (stoma output recycling) is a valuable therapeutic tool in neonates with double enterostomy. The benefits include improving growth and fluid and electrolyte balance and reducing dependence on parenteral nutrition, which in turn reduces liver dysfunction [13–15]. Tube stomas were originally described in short gut patients; however, they have been proven useful in multiple other scenarios with reduced complication rates [16]. Tube stomas proved very valuable in our case as the output of the proximal stoma was quite watery, and the limited fascial surface of the abdomen and the irregular skin made the formation of mature, everted stomas with sufficient area around to apply and manage stoma bags adequately nearly impossible. Tube stomas made recycling possible to the degree that the child could come off TPN even before stoma closure.
Commonly accepted practice in surgical neonates is that energy expenditure usually increases only temporarily for the initial 12–24 hours after surgery [17]. Critically ill premature neonates do not seem to have an increased resting energy expenditure [18, 19]. Routine administration of excess calories may not be warranted in surgically ill premature neonates. This, however, should not be applicable to cases of open wounds, especially laparostomies and EAFs. Our case persistently needed excess calories (over 180 kcal/kg).
An ECF is an abnormal pathway between the intestine and the skin. ECFs in the pediatric age group have a reported mortality of up to 20% [20]. EAFs are a special subset of ECFs. They principally occur in the setting of an open abdomen (laparastomy) due to abdominal compartment syndrome or infection and are defined as communication between a mucosal segment of the intestinal tract and the atmosphere. They are categorized mainly into two entities, deep and superficial, or output amount (high\low) [21, 22]. In a superficial EAF, neither skin nor soft tissue surrounds the opening in the bowel. EAFs represent a significant treatment challenge, especially in premature infants with low birth weight.
Most of the techniques that were proposed over the years for managing EAFs have high failure rates. The technique we implemented of combining negative pressure wound therapy (NPWT) with a stoma bag has shown promise in multiple other studies [21–26]. NPWT eliminates secretions, decreases tissue swelling, promotes granulation tissue growth, and reduces bacterial growth [27–29]. All these mechanisms enhance wound healing. The use of NPWT in our case allowed us to preserve the giant ECF mucosal plate until a time when surgical reconstruction was possible, therefore preventing the development of short gut syndrome, a frequent complication of severe NEC [1–5]. It also allowed the staged restoration of the abdominal domain to accommodate the bowel, starting with the skin, then obtaining a fascial closure at a later stage. We propose that our strategy in using absorbable mesh closure as a stepping stone to final autologous fascial closure is safe and practical in such scenarios and preferable to the usage of a nonabsorbable mesh, which carries a significant infection risk in the presence of such a large exposed mucosal surface.
Conclusion
Optimal surgical management for NEC-related intestinal perforation, particularly in ELBW neonates, is still debatable. Various factors, such as the general condition, prematurity, surgeon expertise, etiology, and the length and location of the affected bowel segment, guide decision-making. EAFs represent a rare and severe complication of laparotomy in such a context. Obviously, the best treatment for EAFs is avoidance. NPWT is a reliable, economical, and efficient transient closure method in neonatal open abdominal wounds and EAFs. It is preferable to avoid extensive bowel resection that could result in short gut syndrome.
Conflict of interest statement
All the authors declare that there is no conflict of interest regarding the publication of this paper.
Funding
This paper did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Patient consent
Written informed consent was obtained from the patient’s guardian for publication of this case report. A copy of the written consent is available.
References
Author notes
Rawan Alhalabi and Ahmad Alsweed are co-first authors.
Muhammad Eyad Ba’ath is the senior author.
- pathologic fistula
- anastomosis, surgical
- necrotizing enterocolitis
- newborn
- infant, premature
- intestinal perforation
- intestines
- reconstructive surgical procedures
- stomas
- surgical procedures, operative
- abdomen
- morbidity
- extremely low birth weight infant
- staged operation
- negative-pressure wound therapy



