-
PDF
- Split View
-
Views
-
Cite
Cite
Annabel Martínez-Sola, Anaí O Reigosa, Rafael Morales-Soriano, Patricia Sánchez-Velázquez, Marta P Damieta, Severe pulmonary toxicity associated with intraperitoneal mitomycin C: a warning from two cases of CRS + HIPEC in different institutions, Journal of Surgical Case Reports, Volume 2025, Issue 7, July 2025, rjaf525, https://doi.org/10.1093/jscr/rjaf525
Close - Share Icon Share
Abstract
Mitomycin C (MMC) is commonly used in hyperthermic intraperitoneal chemotherapy (HIPEC) for peritoneal surface malignancies. While myelosuppression is a known complication, pulmonary toxicity is rare. We present here two cases of acute respiratory distress syndrome (ARDS) following intraperitoneal MMC during CRS-HIPEC. A 61-year-old female developed rapid respiratory failure 36 h postoperatively and died from multiorgan failure; autopsy revealed systemic endothelial injury. A 72-year-old female with pseudomyxoma peritonei developed ARDS on postoperative day 4. After corticosteroid treatment, her condition improved. These cases highlight the risk of severe pulmonary toxicity from intraperitoneal MMC. Clinicians should consider MMC-induced ARDS in the differential diagnosis of postoperative respiratory failure following HIPEC.
Introduction
Mitomycin C (MMC) was historically used in the treatment of gastrointestinal, breast, cervical, and lung cancers. Although the development of newer agents has significantly reduced its intravenous use, MMC remains commonly employed intraperitoneally during hyperthermic intraperitoneal chemotherapy (HIPEC) following cytoreductive surgery (CRS), particularly in conditions such as pseudomyxoma peritonei (PMP) and peritoneal carcinomatosis of colorectal or appendiceal origin [1].
Currently, CRS combined with HIPEC is increasingly used for the treatment of peritoneal carcinomatosis of various origins. Despite its clinical benefits, this approach carries the risk of severe systemic toxicities that are still under-recognized.
The most frequently reported toxicity of MMC during HIPEC is myelosuppression. Neutropenia is observed in up to 39% of patients, although severe neutropenia requiring colony-stimulating factor occurs in ˂10% [2, 3]. Other documented adverse effects include renal impairment and pulmonary infiltrates associated with intravenous administration, however, toxic effects involving other organ systems have been rarely reported. Here, we present two cases of acute respiratory distress syndrome (ARDS) secondary to intraperitoneal MMC during HIPEC, reported from two specialized CRS centres [4, 5].
Case reports
Case 1
A 61-year-old female, diagnosed in 2012 with a pT4aN1 colon adenocarcinoma, underwent adjuvant treatment with FOLFOX and experienced three peritoneal recurrences. In 2021, she was referred to our centre for a locally recurrent tumor involving the duodenum. Cytoreductive surgery revealed a peritoneal cancer index (PCI) of 3. Resection of the fourth portion of the duodenum was performed, followed by HIPEC with MMC (19 mg) and cisplatin (144.5 mg) for 60 min at 40°C–41°C.
Around 36 h post-surgery, the patient developed sudden acute respiratory failure requiring escalating doses of norepinephrine. Imaging studies revealed bilateral interstitial infiltrates consistent with a differential diagnosis of ARDS or acute pulmonary oedema (APE) (Fig. 1). Despite broad-spectrum antibiotics and respiratory support, progressive hemodynamic deterioration ensued, prompting surgical re-exploration. Laparotomy revealed patchy intestinal ischemia likely secondary to multiorgan failure (MOF), culminating in the patient’s death. Autopsy findings revealed systemic endothelial damage with calcium deposition resembling ischemia–reperfusion injury without evidence of sepsis.
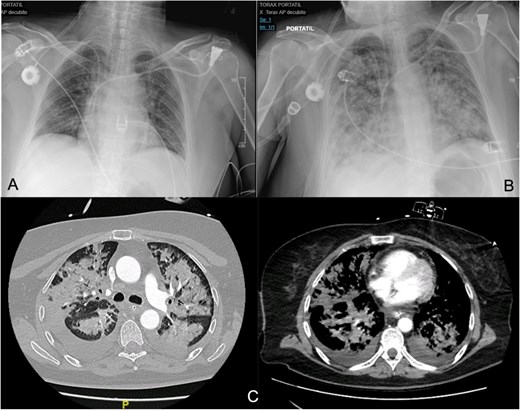
(A, B) Differences between the chest X-ray in the immediate postoperative period and at 36 h after the onset of respiratory failure. (C) The chest CT confirms pulmonary exudates compatible with ARDS (acute respiratory distress syndrome).
Case 2
A 72-year-old female underwent surgery in 2021 for pseudomyxoma peritonei of appendiceal origin with a PCI of 33 and a cytoreduction score of CC-2. She received intraperitoneal MMC (20 mg/m2 for 60 min and 10 mg/m2 for 30 min) diluted in 3 L/m2 of 1.5% dextrose for 90 min at a mean temperature of 42°C.
On postoperative day 4, she developed progressive respiratory failure requiring reintubation. Chest imaging revealed right basal consolidation and left pleural effusion, alongside elevated inflammatory markers (Fig. 2). By day 9, after initiation of weaning, new bilateral patchy interstitial infiltrates were observed on radiographs. Acute pulmonary oedema was excluded based on echocardiography findings and lack of diuretic response, leading to a diagnosis of ARDS. Concurrent pancytopenia was attributed to MMC toxicity. The patient improved progressively following corticosteroid therapy.
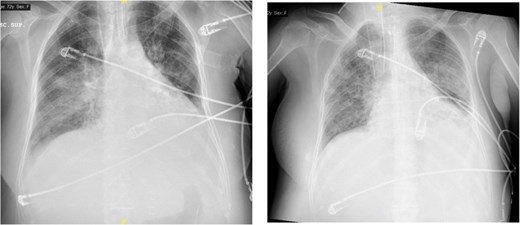
The image on the left shows right basal consolidation and left pleural effusion, which evolves into bilateral interstitial infiltrates in the image on the right.
Discussion
Pulmonary toxicity related to intravenous MMC has been documented since the late 1970s. While rare, its incidence may reach 5%–12%, with interstitial pneumonitis being the most common presentation. Typically, this toxicity manifests insidiously and is considered a diagnosis of exclusion, often responding to oral corticosteroids.
Pulmonary toxicity due to intraperitoneal MMC is significantly less frequent. Abel et al. described delayed symptom onset with resolution by postoperative day 7 after intubation [6]. In contrast, González-Moreno et al. reported a more gradual onset resolving with corticosteroid therapy, without requiring intubation [7].
Both cases presented here involved acute respiratory failure requiring intubation and demonstrated clinical improvement following intravenous corticosteroids, supporting the hypothesis of drug-induced pulmonary toxicity. Autopsy findings in the first case confirmed systemic endothelial damage, predominantly pulmonary.
Following the PRODIGE-7 trial and PSOGI recommendations, many centres specializing in CRS and HIPEC have transitioned from oxaliplatin to MMC for intraperitoneal chemotherapy in pseudomyxoma and colorectal metastases [8, 9].
These cases underscore the potential for fatal pulmonary toxicity associated with intraperitoneal MMC, highlighting the need for its consideration in the differential diagnosis of respiratory failure in affected patients.
Conflict of interest statement
None declared.
Funding
None declared.



