-
PDF
- Split View
-
Views
-
Cite
Cite
Luis F Marcial-Cuevas, Adrian Regalado-Aquino, Francisco Bevia-Perez, Eduardo Lopez-Ortega, Enucleation of insulinoma using laparoscopic distal pancreatectomy with a focus on vascular and splenic preservation: a case report, Journal of Surgical Case Reports, Volume 2024, Issue 8, August 2024, rjae561, https://doi.org/10.1093/jscr/rjae561
Close - Share Icon Share
Abstract
Insulinomas represent <10% of pancreatic tumors. It is a functional neuroendocrine tumor that can cause recurrent and severe episodes of loss of consciousness due to hypoglycemia. Surgical removal is the only curative treatment. The selection of the optimal surgical technique must be individualized for each patient. Currently, there are emerging innovations in less invasive techniques that reduce morbidity. We present the case of a 23-year-old woman who underwent enucleation of an insulinoma localized at the tip of the pancreatic tail after laparoscopic surgery, with a focus on vascular and splenic preservation. The tumor was safely identified during surgery and enucleated without injury to the spleen and adjacent vascular structures or postoperative complications.
Introduction
Insulinoma is a rare neuroendocrine tumor of the pancreas, characterized by hypersecretion of insulin and consequent hypoglycemia [1]. Although they account for <10% of all pancreatic tumors, their clinical and therapeutic impact is significant because of their ability to cause severe and potentially life-threatening symptoms [2]. The clinical presentation of insulinoma can range from mild hypoglycemic symptoms to severe recurrent episodes of loss of consciousness [3]. Proper diagnosis and management of this condition requires a deep understanding of its clinical characteristics as well as a comprehensive evaluation that includes laboratory tests and imaging studies [3].
Laparoscopic pancreatectomy with splenic preservation is crucial for the management of pancreatic disease. Studies have demonstrated the efficacy of these techniques in improving surgical outcomes and preserving splenic function [4–6].
In this case report, we present surgical treatment for insulinoma, highlighting the diagnostic challenges and therapeutic strategies employed. This case emphasizes the role of laparoscopic surgery in managing insulinoma with a focus on vascular and splenic preservation.
Case report
A 23-year-old woman with a history of subclinical hypothyroidism treated with levothyroxine presented with episodes of paresthesia in the lower extremities and nocturnal sweating related to hypoglycemic episodes. Subsequently, she was hospitalized for disorientation, tonic–clonic seizures, and hypoglycemia levels as low as 17 mg/dL. The study protocol was initiated with an abdominopelvic computed tomography (CT) scan with contrast, revealing an ill-defined ovoid structure of 26 mm at the tip of the pancreatic tail with an average value of 65 Hounsfield units (HU; 89 HU in the rest of the parenchyma) and dimensions of 17 mm × 15 mm × 9 mm (Fig. 1). Laboratory analysis revealed the following: 214 ng/dL of insulin-like growth factor type I (IGF-1), 55.40 mIU/L of insulin, 10.1 ng/mL of C-peptide, and >1000 IU/ml of anti-peroxidase antibodies. Endogenous hyperinsulinism secondary to an insulinoma was diagnosed, making the patient a candidate for minimally invasive surgical resolution.
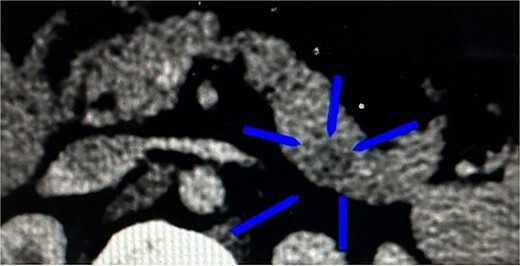
CT of the transverse plane of the pancreas revealed an ill-defined ovoid structure at the pancreatic tail tip.
Surgical technique
The stomach was grasped using atraumatic laparoscopic forceps and retracted upwards. Using a harmonic scalpel, the omentum was divided along the greater curvature of the stomach, the gastrocolic ligament was sectioned, and omentum transcavity was accessed. Subsequently, the transverse colon was retracted downwards, the lower border of the pancreas was identified, and dissection of posterior gastric adhesions of the pancreas was performed.
Once the above procedure was completed, it was dissected along the neck of the pancreas to identify the superior mesenteric vein and splenic vein. An incision was made along the superior border of the pancreas, to the left of the gastroduodenal artery and inferior to the hepatic artery. A plane was created between the portal vein and neck of the pancreas by blunt dissection from the inferior to the superior direction with a blunt-tipped laparoscopic dissector (Fig. 2).
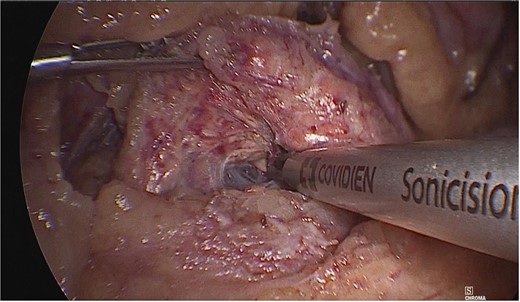
A plane was created between the portal vein and the neck of the pancreas by blunt dissection.
A tumor measuring ~1.5 × 2 cm was identified at the junction of the body with the tail of the pancreas, and dissection of the mesenteric vessels and portal vein was continued. Subsequently, the splenic vein and artery were dissected from the medial to lateral direction at a distance of 2–3 cm. Once released, the neck of the pancreas was divided with an Endo GIA 60 mm endoscopic stapling device (Fig. 3), and a second stapling was performed with an Endo GIA 45 mm (Fig. 4).
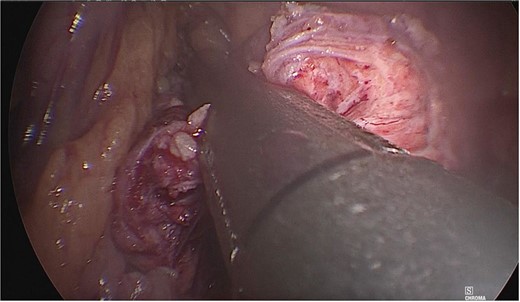
The neck of the pancreas was divided using an Endo GIA 60 mm endoscopic stapling device.
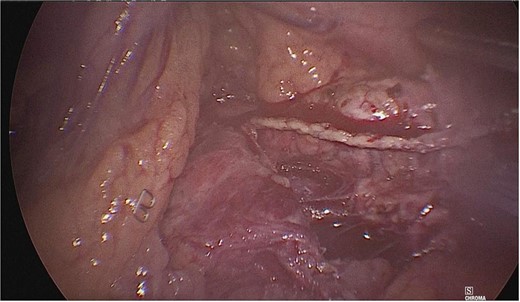
Dissection of the pancreas (body and tail) and splenic vascular structures (arteries and veins; Fig. 5). Intrapancreatic veins were identified. Dissection was performed with a harmonic scalpel of the pancreatic tissue of the vein, taking care not to disrupt it, and the dissection of vascular structures was completed, thus obtaining splenic preservation. The piece was removed, fibrin sealant was applied to the surgical site (Tisseel), and a drain was placed (Fig. 6). The patient had a satisfactory postoperative course and was discharged with a drain in place to be followed up in the outpatient clinic.
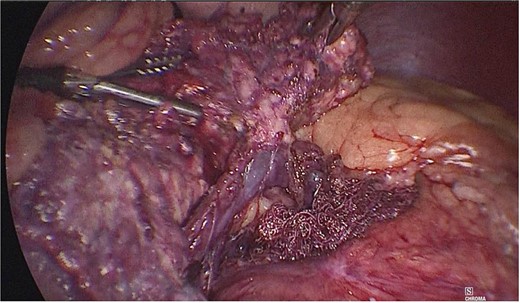
Dissection of the pancreas (body and tail) from splenic vascular structures (artery and vein).
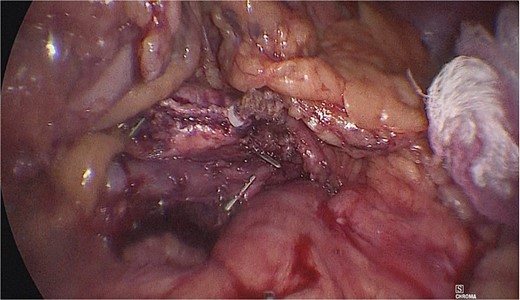
Extraction of the anatomical piece preserving vascular structures.
Discussion
The approach to the treatment of insulinoma has some characteristics, in which surgery is still the only possible curative treatment. The ability to localize a tumor before surgery is crucial for the management of these lesions. Both open and laparoscopic techniques have proven to be effective, but the choice between them depends on several factors, such as the size and location of the tumor as well as the experience of the surgical team [7, 8]. Studies have suggested that laparoscopic distal pancreatectomy may offer significant advantages in terms of postoperative morbidity and recovery time, making it an attractive option in selected cases [9]. However, selection of the optimal surgical technique must be individualized for each patient.
In addition to surgical treatment, the comprehensive management of insulinoma requires multidisciplinary coordination and long-term follow-up. This involves collaboration between endocrinologists, surgeons, radiologists, and pathologists to ensure a comprehensive and personalized approach for each patient [2, 10]. Postoperative follow-up is crucial to detect and address possible recurrences as well as to evaluate and manage possible long-term complications.
Laparoscopic spleen-preserving distal pancreatectomy is associated with lower blood loss and better postoperative recovery in terms of a reduced length of hospital stay. There was a lower risk of overall postoperative complications and wound infections, and no significant differences in the rates of pancreatic fistula or mortality were reported between the two methods [7, 9, 11].
These findings are in line with those of Smith and Johnson [11], who performed a retrospective comparative analysis of laparoscopic pancreatectomy and open surgery in patients with pancreatic adenocarcinoma. They found that laparoscopic pancreatectomy demonstrated similar rates of negative surgical margins and short-term survival rates, but with significant perioperative benefits such as less blood loss and shorter hospital stay.
Conclusion
The management of insulinoma must be based on the decision to perform surgery and a multidisciplinary consensus among physicians, surgeons, and patients. Laparoscopic pancreatectomy with splenic preservation is effective for managing body and tail insulinomas. This surgical technique offers significant advantages in terms of the postoperative morbidity and recovery time. Continuous innovations in this field have highlighted the importance of less invasive and preservative surgical options.
Acknowledgements
Surgeons of the service are recognized for their enthusiasm and passion for teaching.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Funding
None declared.
Informed consent statement
Informed written consent was obtained from the patient for the publication of this report and the accompanying images.



