-
PDF
- Split View
-
Views
-
Cite
Cite
Juanita N Chui, Kejia Wang, Vikram Puttaswamy, Ex vivo repair and autotransplantation for a complex renal artery aneurysm, Journal of Surgical Case Reports, Volume 2023, Issue 7, July 2023, rjad425, https://doi.org/10.1093/jscr/rjad425
Close - Share Icon Share
Abstract
Renal artery aneurysms (RAA) are rare, occurring with an incidence of <0.1%. Open repair remains the mainstay of treatment for anatomically complex aneurysms. Here, we present a case of a large hilar RAA managed with ex vivo reconstruction and heterotopic renal autotransplantation. In this case, the complex anatomy and location of the aneurysm precluded the use of an endovascular approach. In situ repair was deemed unfavorable because of the technical difficulty of the repair with the numerous arterial branches involved, risk of parenchymal injury from prolonged warm ischemic time, restricted surgical field and risk of aneurysm rupture. This case contributes to the literature on laparoscopic nephrectomy, ex vivo repair and autotransplantation as a safe and viable treatment strategy for patients with complex RAA.
INTRODUCTION
Renal artery aneurysms (RAA) are a rare entity [1, 2]. Despite increasing detection rates with the growing availability of angiography and computed tomography, the estimated incidence remains between 0.1 and 1% [1, 2]. Most are discovered incidentally as unilateral and solitary lesions. Patients tend to be asymptomatic, with only a small proportion developing pain, hematuria or renovascular hypertension [2]. However, rupture of RAAs is associated with significant morbidity and an estimated mortality risk of 10% [3]. Current evidence therefore supports elective repair for RAAs exceeding 3 cm in diameter, or in patients with evidence of interval enlargement, complications including dissection or distal embolism, localized signs and symptoms, or additional risk factors, such as hypertension or pregnancy [4–6].
Open surgical repair remains the mainstay of treatment for anatomically complex aneurysms. Herein, we present a case of a large hilar RAA managed with ex vivo reconstruction and heterotopic renal autotransplantation.
CASE DESCRIPTION
A 41-year-old male presented to his primary physician with a history of hypertension and was found to have a large right RAA on duplex ultrasound. He had a smoking history of 20 pack-years and denied any history of abdominal pain, trauma or systemic illness. CT angiography demonstrated a 32 mm fusiform RAA at the hilum of the right kidney, with multiple branches arising from it. His left renal artery appeared normal. He underwent a diagnostic catheter angiogram, which revealed a stenotic web in the right main renal artery and confirmed multiple segmental branches originating from the aneurysm (Fig. 1). Because of the number of branches, endovascular stenting or coiling was not possible without sacrificing a significant proportion of the kidney. Given the potential diagnosis of fibromuscular dysplasia in this patient, preservation of the kidney was important because of the risk of future involvement of the left renal artery. The patient was therefore worked up for an open ex vivo aneurysm repair and heterotopic autotransplantation.
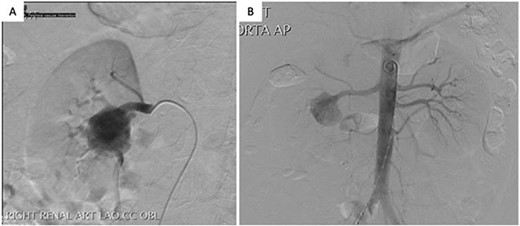
Arterial phase digital subtraction angiography: A) Selective run demonstrating a large RAA arising from the right renal artery bifurcation and involving segmental arteries. B) Aortogram demonstrating both renal arteries.
Transperitoneal laparoscopic nephrectomy was performed with the patient in lateral decubitus position. The right kidney was extracted through a Pfannenstiel incision, placed on ice and perfused with Bretschneider’s histidine-tryptophan-ketoglutarate (Custodiol) solution at 4°C. Back-table bench surgery was performed, and the renal artery was carefully dissected to expose a true aneurysm at the hilum, from which five arterial branches arose. Aneurysmorraphy was performed by excising the anterior wall of aneurysm, away from the origins of the branches with primary closure using 6/0 Prolene suture. All five arterial branches were preserved (Fig. 2). A 5 mm vascular dilator was passed through the main renal artery to break the dysplastic web.
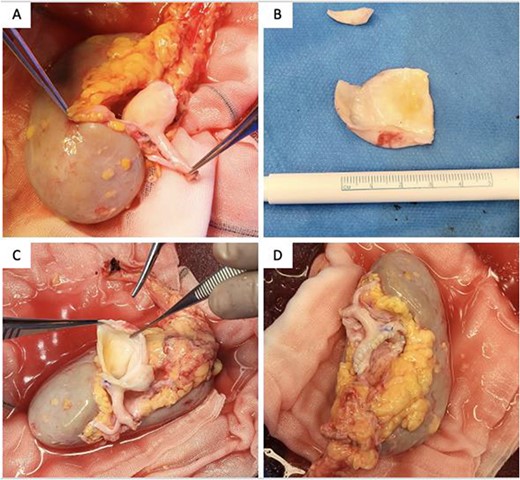
Intraoperative photographs of (A) specimen, (B) resected aneurysmal portion, (C) aneurysmectomy and (D) aneurysmorrhaphy/reconstructed renal artery.
Following ex vivo reconstruction, the kidney was autotransplanted into the right iliac fossa through a Rutherford-Morrison retroperitoneal approach. An end-to-side anastomosis was formed between the renal vein and artery with the external iliac vessels using 5/0 and 6/0 Prolene sutures. Finally, the ureter was reimplanted with an extravesical Lich-Gregoir anastomosis over a double-J ureteric stent. The cold ischemic time was 139 min, and the total warm ischemic time was 37 min. An intraoperative duplex ultrasound scan demonstrated normal renal artery and vein flow, with normal cortical resistive indices between 0.5 and 0.7 and perfusion to the edges of the renal cortex. The patient recovered well and was discharged on postoperative Day 7 on no antihypertensives. Histopathology of the aneurysm wall showed fibrosis and degeneration with no definitive evidence of fibromuscular dysplasia. The patient was well at 2-month follow-up, with no renal impairment and significant improvement to blood pressure control.
DISCUSSION
Treatment for RAAs depends on the location, size and anatomy. Endovascular repair now represents an increasingly preferred modality [7]. The minimally invasive nature of such techniques is advantageous to the patient, but only if the aneurysm can be successfully sealed and the risk of rupture ameliorated. Endovascular options range from using uncovered and covered stents to a combination of occlusive agents, such as coils, plugs and other embolic devices [7]. Aneurysms of the main renal artery can be treated with covered stents if there are adequate seal zones proximally and distally to the aneurysm neck. Another option in this segment of the artery is to use stent-assisted coiling techniques. This treatment option is particularly useful at the hilum of the kidney where the first-order branches arise. Where the renal artery bifurcates into two main branches, the use of open flow-diverting stents in conjunction with coils can achieve adequate sealing of the aneurysm, while still maintaining good perfusion to both branches and associated kidney parenchyma. However, when there are more branches, endovascular options become less attractive, and a third branch at the bifurcation cannot be preserved in this manner; this would need to be treated with occlusion of the smallest branch and acceptance that a section of the kidney would become ischemic. Our case involved five branches of equal size arising from the aneurysm, situated at the renal hilum, such that no endovascular option would allow adequate perfusion to the kidney.
Anatomically complex lesions and those arising at the hilum require open surgical repair [2, 8]. In these cases, in situ repair with aneurysmectomy and reconstruction, with or without a bypass graft, represents the most common approach. However, in situ repair presents several disadvantages; a complex repair can be time-consuming and may prolong warm ischemic time. This can impair long-term kidney function, even if the aneurysm is successfully repaired and circulation restored [9]. Furthermore, aneurysms at the hilum can be challenging to access because of depth and limited field of view. The technical ramifications of this should not be underestimated.
Nephrectomy may be indicated for non-reconstructable anatomy or failed reconstruction [8]. In a young patient, as in our case, this is a treatment of last resort.
Ex vivo renal artery repair and renal autotransplantation is a less common albeit established approach for the management of complex RAAs [6, 10]. The main advantage of this approach is that once the kidney is explanted, it can be perfused with a cold preservation solution, as is regularly used in standard renal transplantation, to safely allow for an extended duration for the repair of complex lesions. In conjunction, the use of a laparoscopic technique for nephrectomy allows for a more minimally invasive approach.
Once the kidney has been explanted and evaluated ex situ, several options are available for open surgical treatment. This includes removal of the aneurysm and reconstruction of the many branches. Another option would be to perform a bypass from the main renal artery to each of its branches. This was not suitable in our case, where five significant branches arose from the main RAA. The basis of treatment revolves around excising the aneurysmal sac and then restoring in line circulation to as many branches as possible. The traditional method is aneurysmectomy followed by aneurysmorrhaphy, either with a primary closure technique or by using a patch. The primary closure technique may be unfavorable if the residual artery is too small, which may cause narrowing of the hilum and loss of some of the branches. The advantage, however, is that this is a simpler method of repair. Alternatively, a patch could be used, with a reduced risk of narrowing of the reconstructed arterial segment. However, if inappropriately reconstructed this can become too dilated, resulting in a neoaneurysm.
CONCLUSION
In the presence of adequate subspecialty surgical expertise, laparoscopic nephrectomy, ex vivo repair and autotransplantation represent a safe and viable treatment option for RAAs, otherwise deemed non-reconstructable and as an alternative to primary nephrectomy.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
The authors received no financial support for the research, authorship and/or publication of this article.
INFORMED CONSENT
Informed consent has been obtained from the patient for publication of this case report and accompanying images.
DATA AVAILABILITY
Data sharing is not applicable to this article as no new data were created or analyzed in this study.



