-
PDF
- Split View
-
Views
-
Cite
Cite
Dai Kimura, Jun Nakayama, Takaomi Hanaoka, Takashi Muraki, Yasunobu Takeoka, Reina Nakamura, Ayako Nakamura, Shugo Takahata, Naoki Ishizaka, Hiroaki Motoyama, Extramedullary hematopoiesis in gastric wall under early gastric cancer in a man with a myeloproliferative disorder: a case report, Journal of Surgical Case Reports, Volume 2022, Issue 7, July 2022, rjac337, https://doi.org/10.1093/jscr/rjac337
Close - Share Icon Share
Abstract
Extramedullary hematopoiesis (EMH) is the proliferation of hematopoietic stem cells outside the bone marrow and often observed in the liver, spleen in association with myeloproliferative disorders. On the other hand, EMH in the gastric wall is extremely rare. We report a rare case of EMH foci coexisting with early gastric cancer, which resulted in severe gastrointestinal bleeding. A 70-year-old male was diagnosed with myelofibrosis 4 years ago and visited our emergency room with a complaint of hematemesis and tarry stools. Upper gastrointestinal endoscopy revealed three early-stage gastric cancers in the lower gastric body and antrum, and biopsy was performed. Persistent bleeding at the biopsy site of the hypogastric lesion led to the consideration of surgical intervention. An open distal gastrectomy was performed. Postoperative histopathological examination revealed the tumor of the lower gastric body had EMH foci associated with myelofibrosis.
INTRODUCTION
Extramedullary hematopoiesis (EMH) is well known as the proliferation of hematopoietic stem cells in sites other than the bone marrow, but EMH in the stomach wall is extremely rare. As far as we know, there are only 11 case reports [1–11]. This is the first case in which EMH of the stomach wall was reported under early gastric cancer. We report a rare case of EMH foci coexisting with early gastric cancer, which resulted in severe gastrointestinal bleeding.
CASE REPORT
A 70-year-old man with transfusion-dependent JAK-2 mutation-positive myelofibrosis presented with complaints of lower abdominal pain, coffee-ground-like vomit and tarry stools, and subsequently underwent emergency endoscopy. The examination revealed a positive red color sign for esophageal varices and 0-IIc early gastric cancer with exudative bleeding in the lesser curvature of the lower gastric body (Fig. 1). Two 0-IIc early gastric carcinomas were found on the anterior and posterior walls of the prepyloric region, respectively, without bleeding. He was also diagnosed with a current infection of Helicobacter pylori. He underwent endoscopic variceal ligation of esophageal varices, biopsy of three gastric cancer lesions and endoscopic hemostasis of the lower gastric body lesion. When a second-look endoscopy was performed to confirm hemostasis the next day, persistent bleeding from the gastric body cancer was observed. Endoscopic hemostasis was reperformed, but complete hemostasis was not achieved. Subsequent blood tests showed progressive anemia, which led to further consideration for surgical hemostasis intervention.
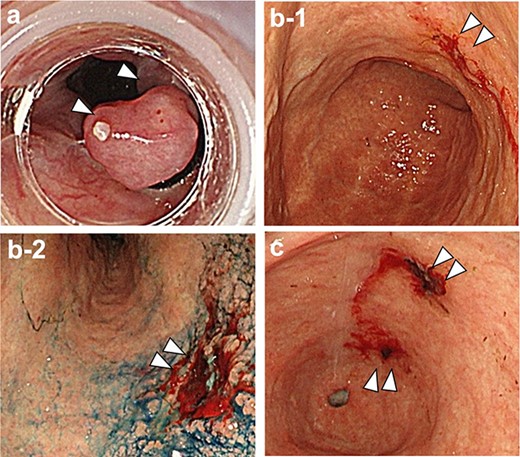
(a) Red color sign positive esophageal varices with white thrombus; (b-1.2) the gastric mucosa showed evidence of Helicobacter pylori current infection, and there was a 0-IIc lesion with exudative hemorrhage in the lesser curvature of the lower gastric body; (c) 0-IIc lesions were also observed on the anterior and posterior walls of the prepyloric region, respectively.
Vital signs were as follows: body temperature: 37.0°C, heart rate: 71 beats/min, blood pressure: 100/60 mmHg. Blood tests showed anemia, thrombocytopenia and coagulation abnormalities.
Abdominal computed tomography (CT) scans showed esophagogastric varices, but no gastric cancer lesion could be identified (Fig. 2).
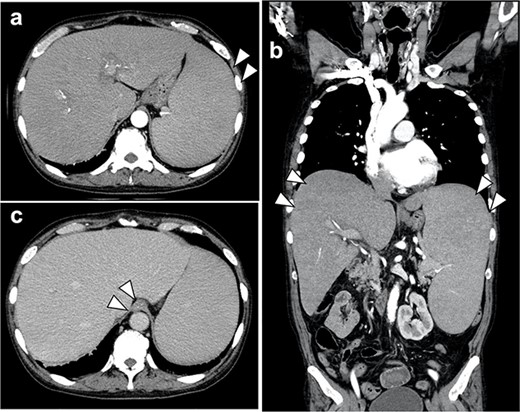
(a, b) Contrast-enhanced CT scan of the abdomen showed marked hepatosplenomegaly due to myelofibrosis; there was no extravasation of contrast medium into the gastroesophagus; no gastric cancer lesion could be identified; (c) the accumulation of contrast media in the lower esophageal wall of the esophagus was observed in the late contrast phase, which was varicose veins.
The patient was diagnosed as having early-stage gastric cancer with unmanageable bleeding, and open distal gastrectomy was performed. Although the operation was difficult due to severe hepatosplenomegaly, the open distal gastrectomy and reconstruction with Roux-en-Y anastomosis were performed as planned. The operation time was 401 min with 360 ml of blood loss.
Macroscopically, three 0-IIc ulcerative lesions in the stomach was observed (Fig. 3).
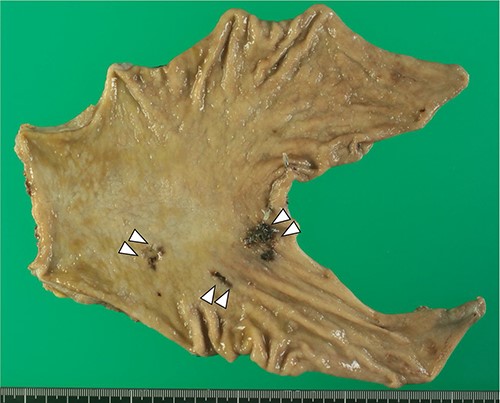
Macroscopically, 0-IIc lesions were found in the lesser curvature of the lower gastric body, and the anterior and posterior walls of the prepyloric region, respectively.
Histopathological examination revealed that these three lesions were all diagnosed as intramucosal adenocarcinoma (well different type, moderately differenced type). Notably in the lower gastric body lesion with persistent hemorrhage, contained a neighboring a submucosal collection of large-nucleated and multinucleated atypical cells and small cells that were clearly different from intramucosal cancer cells (Fig. 4). The large-nucleated dysmorphic cells were CD42b-positive, and the small cells were glycophorin A-positive, identifying them to be megakaryocytes and erythroblast islets, respectively. These findings suggested that EMH due to myelofibrosis was present in the gastric wall just below the tumor, and this may have caused the unmanageable gastric hemorrhage. The pathological stage was pT1aN0M0, Stage I according to the Union for International Cancer Control (UICC) classification.
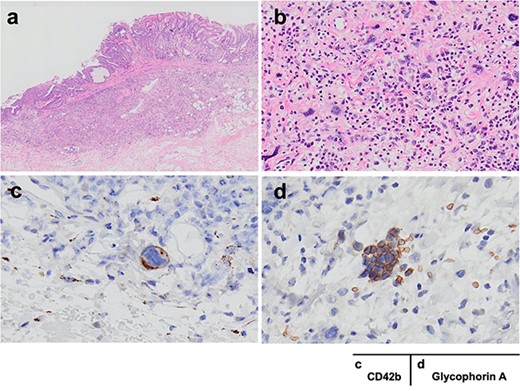
a, b and c, d show HE and immunostaining findings, respectively; (a) the three lesions in the stomach were all intramucosal adenocarcinoma (well different type, moderately differenced type); in the submucosa of the lower gastric body cancer, there were large nuclei, multinucleated atypical cells and small cells that were distinctly different from intramucosal cancer cells; (b) congregation of large nuclei, multinucleated cells and small cells was observed near the tumor cells; (c, d) the dysmorphic cells were positive for CD42b and the small cells were positive for glycophorin A; the cells were judged to be megakaryocytes and erythroblast islets, respectively.
He was discharged 14 days after surgery. After gastrectomy, the frequency of packed red blood cell transfusion was decreased. There was no recurrence of gastric cancer, but he died of fungal pneumonia 7 months after the surgery.
DISCUSSION
EMH in gastric wall is extremely rare, and it can cause upper gastrointestinal bleeding as in this case. Prompt surgical hemostasis is effective for upper gastrointestinal bleeding that endoscopic hemostasis cannot be achieved. Myelofibrosis (MF) is a chronic myeloproliferative disease characterized by bone marrow fibrosis, hepatosplenomegaly due to EMH and the appearance of immature granulocytes and erythroblasts in the peripheral blood. It is classified into primary MF, which occurs without any underlying disease, and secondary MF. The patient was diagnosed with primary MF 4 years before the surgery. Since there was marked hepatosplenomegaly, it can be inferred that EMH was present in the liver and spleen.
EMH often appears as a compensatory response to the disruption of normal hematopoietic function in myeloproliferative disorder (MPD), such as severe anemia and MF. EMH occurs frequently in the liver, and spleen. However, very few cases of non-hepatosplenic extramedullary hematopoiesis (NHS-EMH) have been reported. Most of patients with EMH in the gastric wall in 11 reports were found to be asymptomatic gastric polyps [1–11]. Of these, 10 patients had MPD, but none had EMH just below the mucosa of the gastric malignancy as in this case.
It was reported that 82% of patients with NHS-EMH had hepatosplenomegaly, and about 44% required frequent blood transfusions [12], which as in this case also apply to this case. The prognosis of MF is generally gradual. However, the median overall survival time of MF patients with NHS-EMH has been reported to be 13 months [12]. In fact, this patient also died of fungal pneumonia 7 months after surgery and may have already been in a terminal MF state at the same time of surgery.
Although persistent bleeding associated with gastric cancer is usually caused by ulcers due to advanced cancer, the bleeding ulcer in this case was impossible to stop endoscopically despite the early-stage gastric cancer. EMH foci are rich in blood flow, which often leads to persistent bleeding [13, 14]. The erosion of the mucosal layer caused by gastric cancer may have exposed the EMH foci under the mucosa, causing persistent gastrointestinal bleeding. In addition, the decreased frequency of red blood cell transfusion after gastrectomy suggests that persistent bleeding from gastric cancer may have additionally contributed to the severity anemia in this patient.
Underlying coagulopathy and decreased platelet count may also have contributed to the persistent bleeding in this case. The Japanese Society of Gastroenterology guidelines for peptic ulcers recommend prompt surgical intervention when hemostasis cannot be achieved after two rounds of endoscopic hemostasis or when endoscopic hemostasis cannot be easily achieved [15]. In this case, gastrectomy was performed because endoscopic hemostasis was not possible, and the bleeding ulcer was histopathologically confirmed to be gastric cancer. We believe that the prompt surgical intervention led to the control of bleeding.
In conclusion, when endoscopic hemostasis is difficult, prompt surgical intervention is important. In addition, the presence of EMH foci in the gastrointestinal tract should be considered when unexplained progression of anemia in patients with MPD.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.
References
- hemorrhage
- biopsy
- upper gastrointestinal endoscopy
- gastric cancer
- gastrointestinal bleeding
- hematemesis
- emergency service, hospital
- extramedullary hematopoiesis
- hematopoietic stem cells
- melena
- myelofibrosis
- myeloproliferative disease
- surgical procedures, operative
- bone marrow
- liver
- neoplasms
- spleen
- persistence
- gastric cancer, early
- billroth i procedure
- stomach wall
- histopathology tests
- chief complaint



