-
PDF
- Split View
-
Views
-
Cite
Cite
Kenji Mimatsu, Yusuke Kamitaki, Nobutada Fukino, Laparoscopic transabdominal preperitoneal repair for inguinal hernia in a patient with an adult Still’s disease receiving anti-interleukin-6 receptor monoclonal antibody therapy, Journal of Surgical Case Reports, Volume 2022, Issue 3, March 2022, rjac071, https://doi.org/10.1093/jscr/rjac071
Close - Share Icon Share
Abstract
Tocilizumab, a monoclonal antibody drug against interleukin-6 receptor, has been reported to cause wound infection and delayed wound healing. Therefore, surgeries using artificial materials in patients receiving tocilizumab require careful observation. A 75-year-old man who had been receiving tocilizumab and steroids for the treatment of adult Still’s disease was diagnosed with an inguinal hernia and underwent laparoscopic transabdominal preperitoneal repair. Tocilizumab administration was discontinued for 3 weeks before surgery and was restarted 2 weeks after the surgery. Postoperatively, there was no fever, and the C-reactive protein level was marginally elevated. The patient was discharged from the hospital on the fourth day without any postoperative complications, and no delayed infection or delayed wound healing 1 year after the surgery. A few weeks withdrawal of tocilizumab administration before and after surgery permitted safe laparoscopic hernia surgery using a surgical mesh without infection or delayed wound healing.
INTRODUCTION
Biologic drug therapy has been used to treat various diseases, including inflammatory diseases and cancer. Therefore, there are increasing opportunities for surgery in patients using molecularly targeted drugs. Tocilizumab, a monoclonal antibody drug against interleukin (IL)-6 receptor, is used for rheumatoid arthritis [1], Castleman disease, adult Still’s disease [2] and juvenile idiopathic arthritis [3]. In addition to these diseases, anti-IL-6 receptor antibodies are anticipated to expand their application as a means of controlling organ damage secondary to cytokine storms generated by the induction of inflammatory responses, and have recently been used for coronavirus disease pneumonia [4]. There are concerns that this drug may delay the diagnosis of symptom appearance and cause severe infections as it suppresses the postoperative inflammatory response and immune response that generally occur in surgical treatment [5]. Additionally, delayed wound healing has also been reported to occur [6]. Therefore, careful observation of the prosthesis material implantation site is necessary in patients receiving tocilizumab. Herein, we report a case of laparoscopic transabdominal preperitoneal (TAPP) repair in a patient with an inguinal hernia who had been receiving tocilizumab and steroid therapy for adult Still’s disease.
CASE REPORT
A 75-year-old man with a history of adult Still’s disease for over 10 years was referred to our hospital owing to a right inguinal pain. The patient had been receiving tocilizumab (8 mg/kg, every 2 weeks) and prednisolone (4 mg daily). Abdominal computed tomography revealed a groin hernia in the right inguinal region (Fig. 1). The patient was diagnosed with a right inguinal hernia, and surgery was scheduled. Tocilizumab was discontinued 3 weeks before the surgery. The low dose of prednisolone (4 mg) was continued, and no steroid coverage was performed before or after the surgery.
The patient underwent surgery under general anesthesia with combined the rectus sheath and transversus abdominis plane block. First-generation cephem antibiotics were administered immediately before and after surgery. Laparoscopic findings revealed the hernia was diagnosed as an indirect inguinal hernia (Fig. 2a). The hernia sac was circumferentially dissected and the myopectineal orifice was sufficiently exposed. A polypropylene, lightweight, large pore mesh (Brad© ON FLEX Mesh 15.7 × 10.2 cm, Medicon) was deployed to cover the myopectineal orifice and fixed to the outer border of the rectus abdominis muscle, pubis, Cooper’s ligament and transversus abdominis tendon membrane using absorbable tacker (AbsorbaTack™, Medtronic) (Fig. 2b). The peritoneal flap was closed by continuous sutures using an absorbable thread.
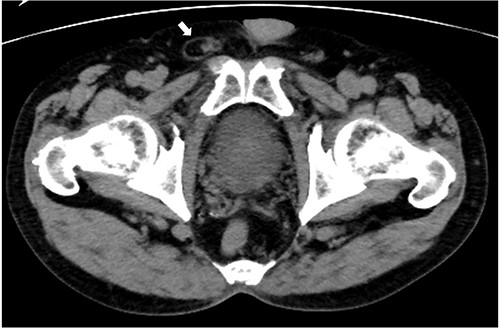
Abdominal computed tomography imaging. A groin hernia in the right inguinal region (arrow).
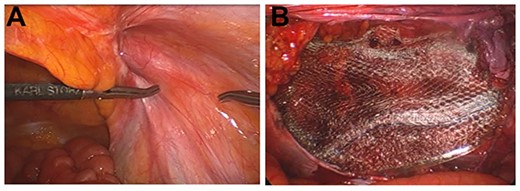
Intraoperative findings. (A) An indirect inguinal hernia, type L-2 according to the classification of the Japanese Society of Hernia. (B) The myopectineal orifice covered with a surgical mesh.
The fever remained below 37°C from the postoperative period until discharge, and the results of blood tests were as follows: white blood cells (WBC) (/μL): 5050, 11 500 and 14 300; C-reactive protein (CRP) (mg/dL): 0.00, 0.56 and 0.25, preoperatively, on the second postoperative day, and the first postoperative week, respectively; WBC levels were high; however, the increase in CRP was mild. The patient discharged on the second postoperative day without complications. Tocilizumab administration was restarted 2 weeks after the surgery. One year after surgery, there was no sign of infection or recurrence of the inguinal hernia.
DISCUSSION
The present case demonstrates two important clinical features. First, laparoscopic repair of inguinal hernia using a surgical mesh was feasible in tocilizumab-treated patients without infection or delayed wound healing. Second, a few weeks of tocilizumab withdrawal is necessary for surgery.
There are some reports on the use of tocilizumab in the perioperative period in orthopedics [6, 7]. Hirano et al. [7] observed fever, WBC, and CRP levels after arthroplasty in patients with rheumatoid arthritis receiving tocilizumab and reported that fever and CRP levels did not increase. This finding suggests that tocilizumab suppresses inflammation that occurs after the surgical invasion, making it challenging to notice the typical signs of postoperative infection. In the present case, there was an increase in the WBC count; however, CRP was hardly elevated. Therefore, we focused on local signs of infection, such as redness and swelling.
Inguinal hernioplasty using a surgical mesh has become a commonly performed operation. However, there are some concerns regarding possible complications, particularly wound infection owing to foreign material in the groin. Mesh infection in inguinal hernia surgery is 0.1%–1.03% [8–11]. Mesh infections in inguinal hernias are uncommon; however, careful observation of the surgical mesh implantation site is necessary as signs of infection are suppressed in surgeries while tocilizumab is being administered. To our knowledge, there have been five reports of nonorthopedic general gastrointestinal surgery during treatment with tocilizumab [5, 12–15]; however, none has reported using surgical mesh for inguinal hernia (Table 1).
Previous reports of nonorthopedic general gastrointestinal surgery during treatment with tocilizumab
| Case . | Year . | Ref. . | Author . | Age . | Gender . | Disease . | Operation disease . | Operative method . | Drug withdrawal period before surgery . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2010 | 14 | Shimizu et al. | 70 | Female | RA | Perfooration due to rectsigmoid colon | Hartmann’s operation | ND |
| 2 | 2015 | 16 | Maruya et al. | 55 | Female | RA | Acute appendesitis | Appendectomy | 1 month |
| 3 | 2017 | 15 | Mitogawa et al. | 65 | Male | RA | Perforation due to sigmoidcolon divelticulum | Hartmann’s operation | ND |
| 4 | 2018 | 17 | Takeda et al. | 73 | Female | RA | Esophageal cancer | Thoracoscopic esopgagectomy | 19 days |
| 5 | 2019 | 5 | Kanagawa et al. | 60 | Male | Castlemann disease | Perforation due to ascending colon cancer | Right hemicolectomy | about 15 days |
| 6 | 2021 | - | Present case | 75 | Male | Still’s disease | Right inguinal hernia | Laparoscopic TAPP repair | 21 days |
| Case . | Year . | Ref. . | Author . | Age . | Gender . | Disease . | Operation disease . | Operative method . | Drug withdrawal period before surgery . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2010 | 14 | Shimizu et al. | 70 | Female | RA | Perfooration due to rectsigmoid colon | Hartmann’s operation | ND |
| 2 | 2015 | 16 | Maruya et al. | 55 | Female | RA | Acute appendesitis | Appendectomy | 1 month |
| 3 | 2017 | 15 | Mitogawa et al. | 65 | Male | RA | Perforation due to sigmoidcolon divelticulum | Hartmann’s operation | ND |
| 4 | 2018 | 17 | Takeda et al. | 73 | Female | RA | Esophageal cancer | Thoracoscopic esopgagectomy | 19 days |
| 5 | 2019 | 5 | Kanagawa et al. | 60 | Male | Castlemann disease | Perforation due to ascending colon cancer | Right hemicolectomy | about 15 days |
| 6 | 2021 | - | Present case | 75 | Male | Still’s disease | Right inguinal hernia | Laparoscopic TAPP repair | 21 days |
RA: Rheumatoid arthritis, ND: not described, TAPP: transabdominal preperitoneal.
Previous reports of nonorthopedic general gastrointestinal surgery during treatment with tocilizumab
| Case . | Year . | Ref. . | Author . | Age . | Gender . | Disease . | Operation disease . | Operative method . | Drug withdrawal period before surgery . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2010 | 14 | Shimizu et al. | 70 | Female | RA | Perfooration due to rectsigmoid colon | Hartmann’s operation | ND |
| 2 | 2015 | 16 | Maruya et al. | 55 | Female | RA | Acute appendesitis | Appendectomy | 1 month |
| 3 | 2017 | 15 | Mitogawa et al. | 65 | Male | RA | Perforation due to sigmoidcolon divelticulum | Hartmann’s operation | ND |
| 4 | 2018 | 17 | Takeda et al. | 73 | Female | RA | Esophageal cancer | Thoracoscopic esopgagectomy | 19 days |
| 5 | 2019 | 5 | Kanagawa et al. | 60 | Male | Castlemann disease | Perforation due to ascending colon cancer | Right hemicolectomy | about 15 days |
| 6 | 2021 | - | Present case | 75 | Male | Still’s disease | Right inguinal hernia | Laparoscopic TAPP repair | 21 days |
| Case . | Year . | Ref. . | Author . | Age . | Gender . | Disease . | Operation disease . | Operative method . | Drug withdrawal period before surgery . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2010 | 14 | Shimizu et al. | 70 | Female | RA | Perfooration due to rectsigmoid colon | Hartmann’s operation | ND |
| 2 | 2015 | 16 | Maruya et al. | 55 | Female | RA | Acute appendesitis | Appendectomy | 1 month |
| 3 | 2017 | 15 | Mitogawa et al. | 65 | Male | RA | Perforation due to sigmoidcolon divelticulum | Hartmann’s operation | ND |
| 4 | 2018 | 17 | Takeda et al. | 73 | Female | RA | Esophageal cancer | Thoracoscopic esopgagectomy | 19 days |
| 5 | 2019 | 5 | Kanagawa et al. | 60 | Male | Castlemann disease | Perforation due to ascending colon cancer | Right hemicolectomy | about 15 days |
| 6 | 2021 | - | Present case | 75 | Male | Still’s disease | Right inguinal hernia | Laparoscopic TAPP repair | 21 days |
RA: Rheumatoid arthritis, ND: not described, TAPP: transabdominal preperitoneal.
There is also a concern that delayed wound healing may occur during tocilizumab administration. In orthopedic surgery in rheumatoid arthritis patients receiving tocilizumab, the incidence of delayed wound healing has been reported to be significantly higher in those undergoing surgical interventions such as foot and spine surgery [6]. In the present case, it was necessary to pay attention to the delayed wound healing caused by tocilizumab administration and the tissue fragility caused by steroids; however, the patient could be operated safely without infection or delayed wound healing by withdrawing only tocilizumab.
Although there is no established theory on the optimal preoperative withdrawal period for tocilizumab, it is considered safe to take at least 2 weeks of withdrawal based on the half-life of tocilizumab for about 6 days and reports of surgical treatment experience in patients with rheumatoid arthritis [6]. Takeda et al. [15] reported that the 19-day withdrawal before esophageal cancer surgery with Castleman’s disease receiving tocilizumab allowed for safe perioperative management without suture failure or infectious complications. In the present case, tocilizumab and steroid were administered before surgery, which was considered to be a risk factor for mesh infection and delayed wound healing. However, the patient could undergo surgery without infectious complications or delayed wound healing by taking tocilizumab withdrawal for 3 weeks preoperatively and 2 weeks postoperatively; however, steroid use was continued owing to the low dose.
In conclusion, we found that inguinal hernia surgery using surgical mesh could be performed safely while patients were receiving tocilizumab by ensuring a rest period before and after surgery.
CONFLICT OF INTEREST STATEMENT
None declared.
CONSENT FOR PUBLICATION
Written informed consent was obtained from the patients for publication of this case report and any accompanying images.
ACKNOWLEDGMENTS
We would like to thank Editage (www.editage.com) for English language editing.
References
- adult onset still's disease
- postoperative complications
- fever
- monoclonal antibodies
- hernias
- hernia, inguinal
- interleukins
- laparoscopy
- patient discharge
- steroids
- surgical mesh
- surgical procedures, operative
- wound healing
- wound infections
- infections
- c-reactive protein measurement
- interleukin-6
- tocilizumab



