-
PDF
- Split View
-
Views
-
Cite
Cite
Julie De Deken, Gregor A Stavrou, Primary adenocarcinoma of an ileostomy in a Crohn patient: a case report, Journal of Surgical Case Reports, Volume 2022, Issue 12, December 2022, rjac609, https://doi.org/10.1093/jscr/rjac609
Close - Share Icon Share
Abstract
Proctocolectomy with ileostomy is an established surgical treatment in patients with inflammatory bowel disease. Adenocarcinoma of an ileostomy is a rare complication in long-term ileostomies. We present the case of a 69-year-old man who presented with bloody stools and a tumour at the ileostomy site 37 years after ileostomy formation. Biopsies confirmed the presence of an adenocarcinoma. Imaging did not show any metastases or advanced local infiltration. A resection of the ileostomy with a broad safety margin and reimplantation of a new Ileostomy was performed. At 18-month follow-up, there is no sign of recurrence. Ileostomy adenocarcinoma in a Crohn’s disease patient is rare with only four cases described in literature. An en-bloc resection and relocation of the ileostomy is the recommended treatment. Education of patients and healthcare professionals on this long-term ileostomy complication is vital for the early diagnosis and treatment.
INTRODUCTION
Proctocolectomy is a recognized treatment in severe cases of inflammatory bowel disease and patients with familial adenomatous polyposis (FAP) [1]. In a small number of these patients, a malignant transformation of the ileostomy can occur. After a review of the literature, 77 cases of malignancy in an ileostomy were found. Only five of these cases occurred in patients with Crohn’s disease (CD) [2–6]. The recognized treatment is en-bloc resection of the tumour AND ileostomy and reimplantation of the stoma [7] .
We present the case of a 69-year-old CD patient, who presented at our centre with a tumorous formation on his ileostomy.
PRESENTATION OF CASE
A 69-year-old man presented at our outpatient department after noticing bloody stools and a tumorous formation on his ileostomy. The mucosa on this lesion was extremely vulnerable and often bled—especially since he was treated with antiplatelet drugs after a recent myocardial infarct.
At the age of 27, the patient was diagnosed with CD. In 1979, a proctocolectomy with ileostomy was performed. Due to the formation of fistulas around the stoma and skin irritation, the position of the stoma was moved from the right to the left lower abdomen in 1984. Since then, the care for his stoma was unproblematic until 6 months ago when he first noticed a swelling.
On clinical examination, there was a mass (6 × 7 cm) with vulnerable mucosa visible as well as a parastomal fistula (Fig. 1). The stoma was functioning well without any signs of obstruction. An endoscopy did not show any further lesions in the small bowel. A biopsy showed an adenocarcinoma without a BRAF-mutation. A CT scan did not present any signs of distant metastasis or local infiltration. Blood tests showed a CEA of 11,9 ng/ml and a CA 19.9 of <5 U/ml.
We continued the Aspirin intake perioperatively as the patient had suffered a cardiac arrest 8 months before. Excision of the Ileostomy en-bloc with the mass and a safety margin of 1 cm was performed. An added explorative laparoscopy showed no signs of peritoneal metastases. The abdominal wall was closed, and a vacuum dressing was inserted subcutaneously. A new ileostomy was created in the right lower quadrant. After a short vacuum therapy, a secondary wound closure was performed. The patient was discharged after 6 days but unfortunately returned with a wound infection (Clavien-Dindo: 3b). After opening the wound and a further brief vacuum therapy, secondary wound closure was achieved.
Histology confirmed the presence of a moderately differentiated, mucinous adenocarcinoma. All resection margins were tumour free. Postoperative multi-disciplinary-tumor board meeting decided for regular follow-up and no additional treatment. After 18 Months of follow-up, the patient remains tumour free.
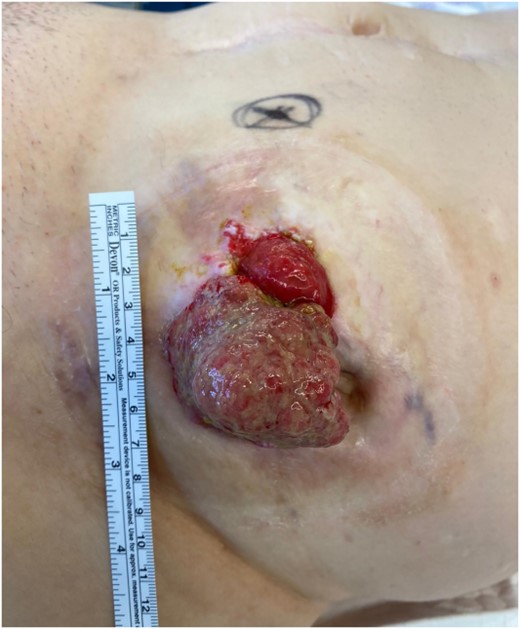
Large, nonobstructive tumour of the ileostomy with vulnerable mucosa.
DISCUSSION
A review of literature showed 77 cases of malignancy of an ileostomy. The most common type of malignancy is adenocarcinoma, followed by squamous cell carcinoma, a handful of cases concerned lymphomas, melanomas or neuro-endocrine tumours. Usually, the primary disease is ulcerative colitis, followed by FAP and CD. The time between ileostomy and tumour diagnosis averaged 30 years. Even though CD patients are known to have an increased risk of small bowel carcinoma, we only found four cases of adenocarcinoma and one of squamous cell carcinoma in ileostomies in patients with CD [2–6, 8].
The pathophysiology of these ileostomy adenocarcinomas is still not fully understood. Mimura et al. have proposed four hypotheses. These include the chronic irritation at the mucocutaneous junction, backwash ileitis, migration of colorectal mucosa into the ileostomy, and colonic metaplasia within the ileostomy mucosa [9].
Diagnosis of these carcinomas is often delayed because they cannot be easily distinguished from a flareup of CD, Ileitis, pyoderma gangrenosum or inflammatory polyps. A biopsy is vital to the diagnosis and should not be delayed—early diagnosis and treatment are essential. Once the diagnosis of adenocarcinoma is confirmed histologically, further staging should follow to exclude the presence of metastases. As for treatment, a resection with wide margins and the relocation of the ileostomy is recommended.
The prognosis for ileostomy adenocarcinoma in non-CD patients is favourable with Metzger et al. [4] reporting an 85% survival rate with surgical resection and only a 15% rate of lymph node metastasis. Small bowel adenocarcinoma in CD patients on the other hand has been known to have a quite poor prognosis with only a 30–69% 3-year survival rate [10, 11]. Due to the small sample size of patients with CD and ileostomy adenocarcinoma, no reliable estimates about their prognosis can be made.
CONCLUSION
Although ileostomy adenocarcinoma is rare, the education of patients and health care workers is vital for the early diagnosis and treatment. We recommend an annual examination of the stoma to exclude the presence of any abnormalities. There should be a low threshold to biopsy any lesions. Resection of the stoma with a wide safety margin and relocation of the ileostomy is the recommended treatment if adenocarcinoma is identified.
DATA AVAILABILITY
The results of the literature review are available from the corresponding author upon request. The relevant patient data are all included in this article.
CONSENT
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
FUNDING
None.



