-
PDF
- Split View
-
Views
-
Cite
Cite
Boyodi Katanga Tchangai, Mazamaesso Tchaou, Fousseni Alassani, Joel Ekoué Amétitovi, Kwamé Doh, Tchin Darre, David Ekoue Dosseh, Giant abdominopelvic desmoid tumour herniated trough perineum: a case report, Journal of Surgical Case Reports, Volume 2021, Issue 8, August 2021, rjab295, https://doi.org/10.1093/jscr/rjab295
Close - Share Icon Share
Abstract
Desmoid tumours are deep aggressive fibromatoses that usually arise in the soft tissues of the limbs or the abdominal wall. Intra-abdominal localisation, rarely occurs and their treatment may be challenging. When necessary, surgery must be personalized to what is achievable in terms of margins while preserving functional outcomes. This condition is illustrated herein with the case of a 40-year-old female presenting an unusually large sporadic desmoid tumour with abdominal, pelvic and perineal involvement. Resection was performed without organ involvement through a combined perineal approach. Tumour resection was macroscopically completed except in the perineum, where the tumour was left (R2 resection) to preserve anal sphincter. Adjuvant treatment with tamoxifen was given to achieve local control. The hormonal treatment was well tolerated, and no recurrence was observed after 36 months of follow-up.
INTRODUCTION
Desmoid tumours are aggressive fibromatoses that account for 3% of soft tissue tumours and 0.03% of neoplasms [1]. They are classified under two clinicopathological entities: the sporadic forms, linked to a somatic mutation of the CTNNB1 gene encoding β-catenin, and desmoid tumours during familial adenomatous polyposis [2]. Regardless of the aetiology, local growth and recurrence are therapeutic problems in desmoid tumours. The treatment options include surgery, radiotherapy, chemotherapy, hormonal treatments and more recent targeted therapies [3, 4]. These treatments remain poorly documented because of the disease rarity and inconclusive results. We report a rare case of a large desmoid tumour with abdominal, pelvic and perineal involvement, which illustrates the complexity of the management plan and the value of tamoxifen as an adjunct to surgery.
CASE DESCRIPTION
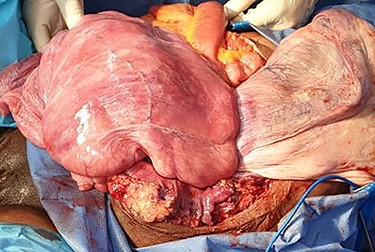
A 40-year-old gravida 4 para 4 was referred to our centre for an abdominal and perineal mass that gradually enlarged over the past 3 years. She complained of abdominal distension, slow transit with 1–2 stools per week, and difficulty in voiding. There was no abdominal pain. She lost 8 kg over the past 3 years. On physical examination, the patient had a large, firm and indolent abdominal mass that emerged through an incisional hernia of the abdominal wall. We also noted a second mass, measuring 40 cm and occupying the internal part of the gluteal region. Its pedicle extended into the pelvis through the right ischiorectal fossa (Fig. 1). Perineal examination revealed a grade IV hysterocele. Computed tomography (CT) showed a huge, well-demarcated solid, homogeneous mass with moderate enhancement occupying the abdomen and pelvis herniating through the perineum (Fig. 2). The core needle biopsy findings were consistent with myxoid neurofibroma. Exploratory laparotomy showed a firm multilobed mass (Fig. 3) that contracted intimate adhesions with the pelvic colon, right ureter and rectum. Resection was performed without organ involvement through a combined perineal approach. Tumour resection was macroscopically completed except in the perineum, where the tumour was left (R2 resection) to avoid anal sphincter injury. The operative specimen weighed 5.842 kg. Histological analysis showed a spindle cell tumour with a myxoid matrix without atypia or mitosis. On immunochemistry, the tumour was negative for PS 100 and CD 117 and positive for nuclear β-catenin and smooth muscle actin. It was diagnosed as a desmoid tumour. During the immediate postoperative period, wound infection and partial dehiscence were noted. Flatus and liquid stool incontinence were also observed. The incontinence completely improved during the 12th postoperative week. Adjuvant treatment with tamoxifen at an initial dose of 150 mg/day and indomethacin (100 mg per day) was given for the incomplete excision (R2). Tamoxifen was tolerated by the patient and continued for 24 months. A follow-up examination 12 months postoperatively documented a complete response. Indomethacin was discontinued after 4 months due to gastralgia. Colonoscopy was performed in the third postoperative month, and there was no polyadenomatosis, confirming the sporadic character of the desmoid tumour. After 36 months of follow-up, no recurrence was observed.
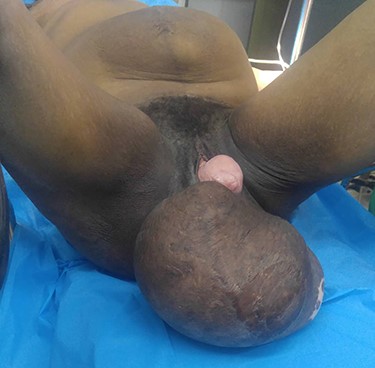
Preoperative view of the patient in supine position showing an abdominal mass and a large perineal lump with hysterocele grade 4.
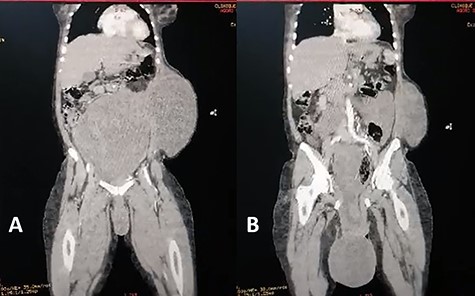
Coronal view of abdominal enhanced CT scan showing: (A) large, well-limited and slightly enhanced abdominopelvic mass. (B) protusion of the abdominopelvic mass trough perineum.
DISCUSSION
Most desmoid tumours arise in the soft tissues of the limbs or the abdominal wall [1]. Intra-abdominal localisation, as reported here, rarely occurs and accounts for 11% of cases [3]. Both sexes can be affected, but it most commonly affects young women [2], similarly to our observation. Intra-abdominal desmoids frequently present as progressively enlarging abdominal masses [3, 5]. In rare cases, the mass is significantly enlarged. In a study by Xiao et al., only 1 of 16 patients had a mass whose diameter was >20 cm. Although large desmoids have been previously reported [4], none had abdominal pelvic and perineal features. The diagnostic workup for large desmoid tumours is challenging. In almost half of the cases, the patients previously underwent surgery [3, 5]. However, ruling out recurrence is difficult for cancer patients with a history of surgery. Despite the lack of specific features, imaging remains essential in the diagnosis, staging, and follow-up. In this case, the CT scan detected a solid well-demarcated mass that was iso- or hypodense compared to muscle, which is the most common feature [6]. The diagnosis is mainly based on histology, characterized by a spindle cell tumour without atypia or mitosis with a collagenous or myxoid stroma [1, 2]. Immunohistochemistry is needed to rule out differential diagnoses, including low-grade sarcoma, benign fibroblastic proliferation, and reactive processes. Nuclear β-catenin is a good marker even though it is non-specific [2].
The optimal treatment of patients with desmoids remains controversial. Surgery, radiation, chemotherapy and hormone therapy do not guarantee tumour clearance and disease-free survival [1, 7]. Negative margin resection has been considered as the gold standard [7]. Complete resection is not always possible due to organ involvement. In our case, the tumour affected the anal sphincter. There was a gap between our case and the evolution of treatment guidelines for desmoids. Abstention is currently the first-line option based on the spontaneous regression observed in patients [2, 8]. This option was not possible in our patient because of the large tumour size and the risk of complications. In symptomatic or complicated cases, resection is the most frequent treatment option for intra- abdominal desmoids. However, nearly 30% of cases resulted in incomplete resection, as in this case [2–4]. This may be questionable, considering the risk of aggressive regrowth or progression. The role of positive margin resection in disease progression remains unclear [7]. They may not increase the risk of recurrence [9]. However, 30–40% of cases recurred even with complete excision [1], so adjuvant treatment should be considered. Hormonal treatment with tamoxifen combined with non steroidal anti-inflammatory drugs (NSAIDs) is the most accessible, cheapest, and less toxic adjuvant modality [7]. Given these, it should be considered as a first-line option despite the low complete response rate.
CONCLUSION
The treatment of desmoid tumours underwent a paradigm shift that favours the non-operative approach. However, intra-abdominal locations pose complex therapeutic problems which warrant personalised treatment, including surgery. Hormonal treatment following partial resection resulted in complete and long-lasting response.
ACKNOWLEDGEMENTS
Informed consent: written informed consent was obtained from the patient for the publication of images and clinical data in this article.
CONFLICT OF INTEREST STATEMENT
None declared.



