-
PDF
- Split View
-
Views
-
Cite
Cite
Nawaf M Alwagdani, Sahar M Alnefaie, Arif Kurshid, Hanady Kewan, Atypical ductal hyperplasia of the breast mimics breast cancer presentation treated surgically: a case report and literature review, Journal of Surgical Case Reports, Volume 2021, Issue 7, July 2021, rjab325, https://doi.org/10.1093/jscr/rjab325
Close - Share Icon Share
Abstract
Atypical ductal hyperplasia (ADH) is considered a benign lesion with increased malignant potential. ADH represents ~3% of total benign breast biopsy results. A 60-year-old woman with no family history of breast cancer presented with multiple painless lumps in the right breast and palpable right axillary lymph nodes. Ultrasonography and mammography showed typical features of breast cancer. However, core needle biopsy revealed ADH without infiltrating malignancy. Based on a multidisciplinary decision, a right simple mastectomy with sentinel lymph node biopsy was chosen as the best treatment plan. The postoperative histopathology report showed the spread of ductal carcinoma in situ (DCIS) in all quadrants without any evidence of invasive cancer. It is challenging to obtain an accurate diagnosis of clinically palpable and multicentric ADH or DCIS based on preoperative radiological and histological evaluation, especially when dissonance between these two evaluation modalities exists.
INTRODUCTION
A papilloma is divided into four types by the World Health Organization: (i) benign, (ii) atypical ductal hyperplasia (ADH), (iii) ductal carcinoma in situ (DCIS) or (iv) malignant [1]. ADH is considered a benign lesion with increased malignant potential. [2]. It has been reported that 3% of total benign breast biopsies are ADHs [3]. In terms of histological features, ADH and low-grade DCIS share common features, yet DCIS is more extensive and has an 8–10 relative risk of later breast malignancy [4–6].
We present a case of a woman with a preoperative disagreement between her radiology report (malignant lesions) and the histopathology biopsy result (ADH). Later, DCIS was detected on the final histopathology.
CASE REPORT
At the end of 2018, a 60-year-old woman presented to our clinic as she had incidentally felt multiple painless lumps in her right breast but no nipple discharge. Bilateral breast examination revealed no skin or nipple changes and multiple right breast lumps. The largest lump was located in the retro-areolar area. Bilateral axillae examination revealed small, mobile and palpable right axillary lymph nodes.
Ultrasonography (US) showed multiple hypoechoic solid lesions scattered in the right upper outer quadrant. The two most suspicious lesions measured 7 × 5 and 5 × 4 mm, and the rest were <1 cm. One of these lesions showed a microlobulated border, whereas the other had an indistinct border. Based on the Breast Imaging Reporting and Data System (BI-RADS), they were classified as BI-RADS IV A (Fig. 1). Mammography showed innumerable dense lesions in the upper outer quadrant and retro-areolar area. Some had indistinct borders, whereas others were serrated. Linear and cluster microcalcification foci also appeared. These findings led to a classification upgrade to BI-RADS IV C (Figs 2 and 3). The first core needle biopsy (CNB) indicated papilloma with ADH from one lesion and degenerated cyst content with histocytes from the other lesion without any signs of infiltrated malignancy. The patient was given the option of excisional biopsy; however, she opted for alternative medicine.
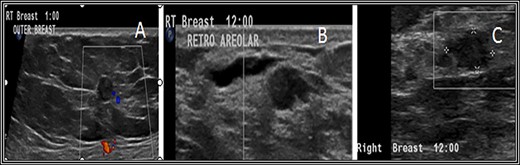
Ultrasound (US) scan of the right breast revealed innumerable hypoechoic breast lesions. (A) Some of them appear round with well-defined regular borders. (B) Others show microlobulated borders with additional dilated duct beside. (C) Others show indistinct borders.
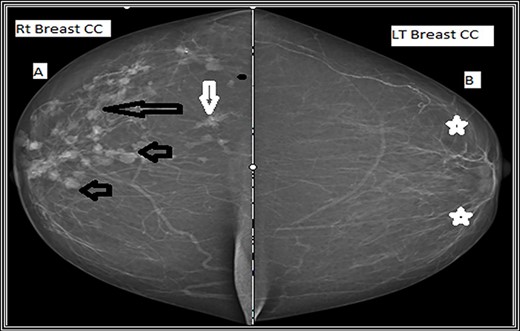
Craniocaudal mammographic view. (A) The right breast shows multiple variable size dense mass lesions, showing regularly lobulated borders (short black arrows), indistinct borders (long black arrow) and speculated outer borders (short white arrow). (B) The left breast shows scattered areas of fibroglandular densities (white stars).
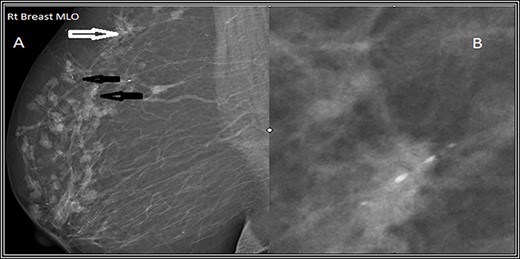
Mediolateral oblique (MLO) mammographic view of the right breast. (A) Shows multiple foci of microcalcifications arranged in linear distribution (black arrows) and others in clusters (white arrows). (B) Spot magnification view of the most suspicious lesion shows linear fine microcalcifications.
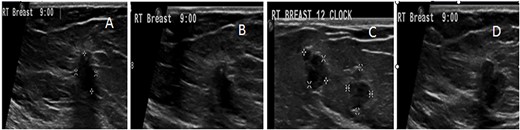
US follow-up after the first biopsy revealed several findings: (A) the most outer lesion can be seen at 9:00 and became vertically oriented inside the breast parenchyma with irregular serrated margins; it infiltrated the adjacent breast parenchyma and the deep posterior acoustic shadow. (B) A tiny microcalcification can be seen at its upper pole. (C and D) An enlargement of the previously seen mass lesions after a second biopsy in which all of them have microlobulated borders and are longitudinally oriented inside the breast tissue can be observed.
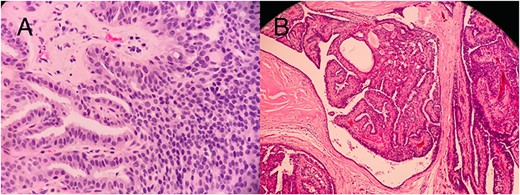
Histopathology slides (A) CNB showing papilloma lined by a double-layer epithelium with foci of ADH without invasion. (B) Postmastectomy, showing a solid DCIS.
Five months later, she returned for medical treatment, and US follow-up revealed some changes in the two most suspicious lesions; one of them had become longitudinally oriented with posterior acoustic shadowing, irregular infiltrating serrated borders, and tiny microcalcifications inside. The other lesion was mildly enlarged with microlobulated outer borders (Fig. 4). Typical malignant US features were found; so the outer lesion was upgraded to a BI-RADS V classification (Fig. 4). A decision was made to repeat the biopsy, which showed ADH with no infiltrating malignancy (Fig. 5). The patient was counseled regarding her diagnoses and the proposed management plan, which was open excision of the two highly suspicious lesions, yet she insisted on having a right simple mastectomy. The risks and possible postoperative complications of the chosen type of operation were explained to her and her family, and they agreed to proceed. Due to the high malignant potential of right breast lesions and the patient’s desire for simple mastectomy, a right simple mastectomy with sentinel lymph nodes biopsy was performed.
The postoperative histopathology report showed the spread of right breast DCIS and microcalcifications through all breast quadrants (Fig. 5). Unintentionally, seven axillary sentinel lymph nodes, which were matted and aggregated together, were found to be reactive without any evidence of malignancy. The patient was reassessed at 2 weeks and at the 1- and 2-year follow-up visits and was found to have no evidence of any local recurrence in the right mastectomy scar.
DISCUSSION
Intraductal papilloma (IDP) is known to fall under the category of proliferative breast disease with a risk of breast cancer development of 1.5- to 1.9-fold [7]. According to some studies, pathologists lack consensus about the differentiation between ADH and DCIS [8], whereas others show consensus is possible when pathologists follow standardized criteria [9]. The false-negative rate of 14-G US-guided CNB ranges from 0.1 to 3.7%; thus, imaging-guided breast biopsy success depends on biopsy technique and definitive agreement between clear postbiopsy imaging and pathology [10].
Familiarity with the BI-RADS classification is a crucial step for correctly determining imaging–pathology similarity for all accessible modalities [11, 13]. According to the new BI-RADS update, the likelihood of malignancy within category 4 (suspicious) ranges from 2 to 95% [11]. Category 4 is subdivided (A, B and C) in the fourth edition of BI-RADS [11], and this subcategorization can estimate the likelihood of malignancy; however, no established objective criteria and no clear interobserver agreement for estimating malignancy in this category exist [11]. A benign core biopsy result is considered a category 4A lesion, unlike category 4C lesions in which a benign core biopsy result would be considered discordant [11]. Imaging–pathology disagreement has been reported as 2–19.2% [12]. Benign lesions can mimic malignancy on US; however, after US-guided CNB, 4–30.9% of discordant lesions were confirmed as cancer after subsequent excision [12].
When concern regarding a dissonant benign biopsy exists, immediate communication within the healthcare team about the discrepancy and best biopsy method is necessary [11]. On the other hand, the high-risk/borderline category is not malignant but presents a high lifetime malignancy risk along with the ongoing controversy of whether surgical or oncological treatment is the appropriate management [13]. According to the Mayo Clinic, a large cohort study found an accumulative risk of breast cancer development in those with high-risk lesions (such as ADH), and 25 years after the first biopsy, malignancy developed in 30% of the women with these lesions [2]. Management options should be individualized based on the patient’s overall status [13]. When ADH is identified on CNB, it carries a high risk of associated malignancy. Therefore, CNB requires a correlation with the clinical findings and the imaging to either follow an established risk-based plan of follow-up or to exclude malignancy by surgical excision. For the same reason, when ADH is found, it is often surgically excised [14]. In our case, the patient was diagnosed with right breast ADH on the first biopsy and refused surgery. After reevaluation, multicentric suspicious lesions with ADH were found, and a mastectomy was performed as per the patient’s wish.
The clinical challenge was the precise diagnosis and follow-up of this patient’s many lesions. The presence of multicentric or large ADH or DCIS lesions is considered an indication for mastectomy and sentinel lymph node biopsy. This case is unique in terms of multicentric suspicious lesions with palpable ipsilateral axillary lymph nodes. A thorough discussion with the patient and her family about the diagnostic uncertainty and a plan for a mastectomy with delayed reconstruction was chosen.
CONCLUSION
It is challenging to obtain an accurate diagnosis of clinically palpable and multicentric ADH or DCIS based on preoperative radiological and histological evaluation, especially when dissonance between these two evaluation modalities exists. In such cases, we believe that the management plan should be discussed thoroughly in a multidisciplinary approach, individualized to each patient, and that the patient should be counseled.
CONFLICT OF INTEREST STATEMENT
None declared.
CONSENT FOR PUBLICATION
Written informed consent was obtained from the patient for publication of this report and accompanying images.



