-
PDF
- Split View
-
Views
-
Cite
Cite
Quang Hong Le, Vuong The Mai, Malignant phyllodes tumor with synchronous metastases to axillary lymph nodes, lung at the presentation: a case report and literature review, Journal of Surgical Case Reports, Volume 2021, Issue 7, July 2021, rjab302, https://doi.org/10.1093/jscr/rjab302
Close - Share Icon Share
Abstract
Phyllodes tumors are rare mesenchymal tumors of breast. Malignant phyllodes tumors can develop metastases to distal organs with spreading hematogenously to most frequent sites as lungs, bone and brain. Regional lymph node enlargement is common but metastasis to lymph node is a very rare phenomenon with prevalence about 1.1–3.8%. In this report, we introduce a case of malignant phyllodes tumor of breast in 57-year-old female patient. Synchronous metastases to axillary lymph nodes and lung were found at the presentation. She was treated with mastectomy combined with axillary lymph node dissection and adjuvant chemotherapy. Axillary lymph node dissection can be considered in case of proven metastatic or suspiciously palpable lymph nodes.
INTRODUCTION
Phyllodes tumors are rare mesenchymal tumors accounting for 0.3–0.9% of all breast neoplasms [1]. Based on stromal characteristics as cell atypia, excessive growth, mitosis and tumor boundary, the World Health Organization categorizes phyllodes tumors as benign, borderline and malignant [2]. Approximately 9–27% of patients with malignant phyllodes tumor have metastasis to distal organs with spreading hematogenously to most frequent sites as lungs, bones and brain [3]. Regional lymph node enlargement is common but rarely metastasized by phyllodes tumor. Only few cases of malignant phyllodes with lymph node metastases have been reported in literature [4]. In this report, we introduce a case of malignant phyllodes tumor of breast with synchronous metastases to axillary lymph nodes and lung at the presentation.
CASE PRESENTATION
A 57-year-old female presented with right breast mass. She noticed rapid growth about 3 months prior to admittance to hospital. There was no family history of breast cancer. Physical examination revealed a mass measuring 6 × 8 cm with solid consistency, movable to the chest wall and normal nipple areola complex. Several enlarged and mobile lymph nodes were clinically found in the right axilla; the contralateral breast, axilla and neck examination were normal.
Breast ultrasound showed a heterogeneous mass occupying the right breast with solid and cystic components and enlarged right axillary lymph nodes measuring 0.5–1 cm. Fine needle aspiration of tumor showed as suspicious carcinoma.
Core tissue biopsy revealed prominent mixed fibroepithelial and stromal proliferation, suggestive of phyllodes tumor.
CT scan of the chest revealed a large tumor involved whole right breast tissue (Fig. 1), bilateral pulmonary metastasis without mediastinal adenopathy (Fig. 2) and axillary lymph node enlargement measuring 1.5 cm with central necrosis.
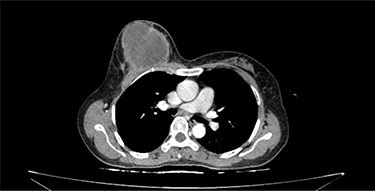
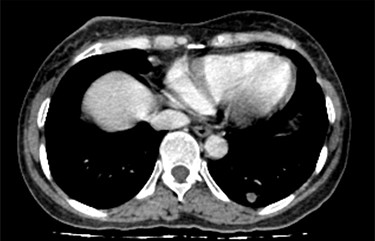
She was diagnosed as metastatic phyllodes tumor and underwent modified radical mastectomy with axillary dissection. Grossly, the specimen measured 6 × 10 × 16 cm with nipple areola complex, and the tumor was identified as ulcerated part measuring 6 × 8 × 8.5 cm with a hard density area as bone. Additionally, grossly identified enlarged axillary lymph node measured 1 × 2 cm. The histopathological findings were suitable with malignant phyllodes tumor with osseous and chondroid metaplasia (Fig. 3). Tumor was positive for Vimentin, CKAE1/AE3 and negative for Desmin, CD34 and P63 in immunohistochemistry sample. Two out of 10 axillary nodes were metastasized. Her post-operative recovery was uneventful with well healing wound. In the next step, she was treated with chemotherapy with Docetacel–Gemcitabine regimen.
DISCUSSION
Early diagnosis of phyllodes tumor helps to make an accurate procedure of treatment, including surgery. However, no clinical, ultrasonic and mammographic indicators have been identified that allow to differentiate between benign and malignant tumors, or phyllodes tumor and a fibroadenoma. Fine needle aspiration (FNA) is not able to get high specificity to diagnose phyllodes tumor but can be helpful in differentiating benign from malignant phyllodes tumors. The value of FNA in the diagnosis of phyllodes tumor is still limited with a high false negative rate and low overall accuracy about 63% [5]. Core needle biopsy offers additional features that can help distinguish phyllodes tumors from fibroadenomas, such as increased stromal cellularity, stromal cell atypia, mitoses and relative proportion of stroma to epithelium.
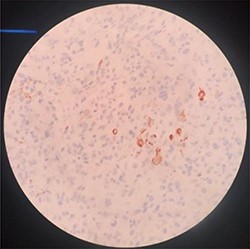
H-E sample of malignant phyllodes tumor with osseous and chondroid metaplasia.
Overall, 10% of phyllodes tumor cases develop distant metastases and occur in ~25% of patients with malignant tumors; 3–12% of metastasis cases occur at the time of presentation or late as more than 10 years. Most distant metastases develop without evidence of local recurrence. Metastasis occurs hematogenously to commonest sites as the lungs (66%), bones (28%) and brain (9%) and, in rare instances, to the liver and heart [6]. However, metastasis to regional lymph node is a rare phenomenon. Since most sarcomas metastasize hematogenously, this finding may explain why axillary metastasis is so rare. In Norris and Taylor’s study of 94 patients, metastasis to the axillary lymph nodes was found in only 1% of all cases and in 17% of cases with enlarged lymphadenopathy [7]. In a study conducted by Gullett and colleagues, 9% of 1035 cases of patients with phyllodes tumor were subjected to an axillary sampling of more than 10 lymph nodes but nodal involvement was documented only in nine patients [8]. Therefore, clinical adenopathy can be a result of reactive hyperplasia due to tumor necrosis. The incidence of axillary lymph node involvement in these series ranged from 1.1 to 3.8% [9].
Surgical management plays the most important role in treatment of phyllodes tumors. If diagnosed preoperatively, surgical therapy is aimed at complete excision of the tumor with at least 1-cm margins particularly in the borderline and malignant phyllodes tumors in the form of either lumpectomy or mastectomy, depending upon the tumor size to the breast. In our case, the tumor measuring 6 × 8 cm involved whole breast tissue, so it was unable to wide resection and mastectomy was indicated. The role of axillary lymph node dissection in malignant phyllodes tumors is controversial. Majority of reports recommend against axillary lymph node dissection due to the low risk of lymph node involvement, except in patients with proven metastatic lymph nodes or in clinically palpable axillary lymph nodes. Our patient had palpable axillary lymphadenopathy, so she underwent axillary dissection.
The role of adjuvant radiotherapy and chemotherapy remains uncertain, but results from using radiotherapy and chemotherapy for soft tissue sarcomas suggest that it can be considered in cases of malignant phyllodes tumors. Doxorubicin-based adjuvant chemotherapy is recommended for breast sarcomas’ first-line treatment [10]. The NCCN recommends that treatment in patients with phyllodes tumor follows the algorithm outlined in the NCCN Soft Tissue Sarcoma Clinical Practice Guidelines and several drugs (Docetaxel + Gemcitabine, Pazopanib or Trabectedin) may be used [11].
CONCLUSION
Only a few cases of phyllodes with lymph node involvement have been reported in the literature. We introduce a case of malignant phyllodes tumor of breast with synchronous metastases to axillary lymph nodes, lung at the presentation. A procedure of mastectomy combined with chemotherapy was applied. In clinical practice, axillary lymph node dissection still can be considered in case of proven metastatic lymph nodes or clinically palpable nodes.
ACKNOWLEDGEMENTS
The author is sincerely grateful to the patient and colleagues in the Department of Pathology, Department of Diagnostic Imaging at Vietnam National Cancer Hospital.
AUTHORS’ CONTRIBUTIONS
Conceptualization, Q.H.L.; Methodology, Q.H.L.; Writing—Original Draft Preparation, V.T.M.; Writing—Review and Editing, Q.H.L.; Visualization, Q.H.L.; Supervision, Q.H.L.
CONFLICT OF INTEREST STATEMENT
The author states no conflict of interest.
FUNDING
This research received no external funding.



