-
PDF
- Split View
-
Views
-
Cite
Cite
Shunichi Ito, Yutaka Takahashi, Takuji Yamada, Yosuke Kawai, Kei Ohira, Xanthogranulomatous appendicitis with elevated tumor marker misdiagnosed as cecal cancer: a case report, Journal of Surgical Case Reports, Volume 2021, Issue 7, July 2021, rjab274, https://doi.org/10.1093/jscr/rjab274
Close - Share Icon Share
Abstract
Xanthogranulomatous inflammation is an uncommon chronic inflammatory disease that develops most often in the kidneys and gallbladder. However, xanthogranulomatous appendicitis 45eXA is rare. Herein, we present a case of XA, with an elevated tumor marker, misdiagnosed as cecal cancer. A 76-year-old woman was referred to our hospital. Carbohydrate antigen 19–9 (CA 19–9) levels were elevated. By computed tomography and magnetic resonance imaging, we diagnosed as suspected cecal cancer and performed laparoscopic-assisted ileocecal resection. The pathological diagnosis was XA. Her CA19–9 level decreased to within normal limits. XA is a condition that results from an unusual healing pattern of appendicitis. However, the underlying mechanisms are still unclear. This is the first case of XA with elevated CA 19–9 levels. In this case, XA may have had the potential for malignancy. Our case report can aid in the understanding of these rare cases and, as a result, improve their prognosis.
INTRODUCTION
Xanthogranulomatous inflammation (XI) is an uncommon chronic inflammatory disease that develops most often in the kidneys and gallbladder [1]. A retroperitoneal xanthogranuloma was first described by Oberling in 1935 [2]. Subsequently, XI has been reported in other organs, including the lungs, pancreas, liver, ovary, urinary bladder and orbit [3–5]. The xanthogranulomatosis of the alimentary tract was first described by Schwarzmann in 1955 [6]. In particular, xanthogranulomatous appendicitis (XA) is rare. Herein, we present a case of XA, with an elevated tumor marker, misdiagnosed as cecal cancer.
CASE REPORT
A 76-year-old woman visited a previous hospital with right lower abdominal pain lasting for ~1 month. She underwent computed tomography (CT), was diagnosed with a cecal tumor and was referred to our hospital. Her medical history included atrial fibrillation, cerebral infarction, hyperlipidemia and hypertension. On physical examination, she had mild tenderness in the right lower abdomen.
Laboratory tests revealed that her white blood cell count and carcinoembryonic antigen (CEA) levels were within normal limits (8600/μl and 2.4 ng/ml, respectively). However, C-reactive protein and carbohydrate antigen 19–9 (CA 19–9) levels were elevated (17.73 mg/dl and 87.8 U/ml, respectively). Colonoscopy showed swelling of the Bauhin valve and an elevated tumor of the terminal ileum (Fig. 1), but the biopsy specimen showed no malignancy. Abdominal contrast-enhanced CT detected a partially high-density tumor (diameter: 90 × 70 mm) in the cecum with some peripheral lymphadenopathy (Fig. 2). Magnetic resonance imaging (MRI) revealed a tumor (diameter: 60 × 40 mm) with thickening of the appendix wall near the cecum (Fig. 3). Although her pain resolved with conservative therapy, we diagnosed as suspected cecal cancer based on the imaging findings and elevated tumor marker levels.
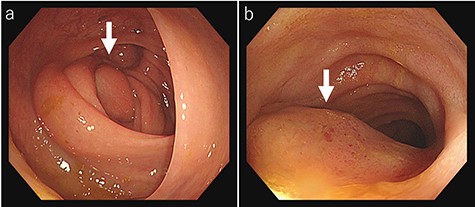
Colonoscopy findings colonoscopy reveals swelling of the Bauhin valve (a) and an elevated tumor of the terminal ileum (b).
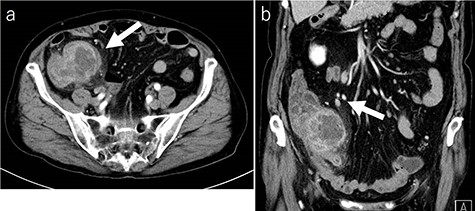
CT findings (a: axial image in the artery phase; b: coronal image in the artery phase). Abdominal contrast-enhanced CT showing a partially high-density tumor (diameter: 90 × 70 mm) at the cecum and some peripheral lymphadenopathy (shown by arrow).
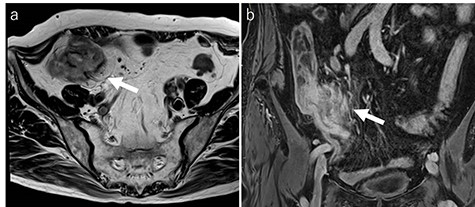
MRI findings (a: T2-weighted axial image; b: contrast enhanced T1-weighed coronal image). MRI showing a tumor (diameter: 60 × 40 mm) with thickening of the appendix wall near the cecum (arrow).
Therefore, surgery was performed. Intra-operatively, inflammation was observed in the terminal ileum (Fig. 4). We performed laparoscopic-assisted ileocecal resection with D3 lymphadenectomy. The resected specimen exhibited a yellowish change near the root of the appendix (Fig. 5). Microscopically, a nodular lesion with unclear boundaries was detected from the appendix root to the ileocecum, formed by fibrous cells, foamy histiocytes, foreign body giant cells and inflammatory cell infiltration (Fig. 6). There were no Michaelis–Gutmann bodies or malignancies. Based on these findings, the diagnosis was XA. No post-operative therapy was administered, and the patient remained uneventful for 20 months following surgery. The CA19–9 level decreased to 22.7 U/ml a month later and was within normal limits 20 months post-operatively.
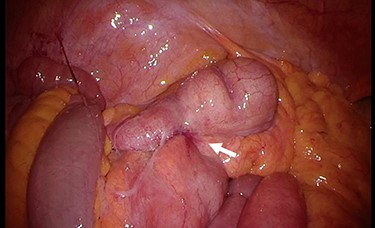
Intra-operative findings. Inflammation is observed at the terminal ileum (arrow).
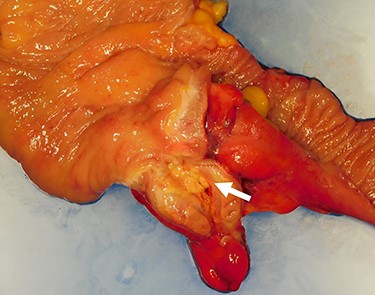
The resected specimen. The resected specimen showing a yellowish change near the appendix root (arrow).
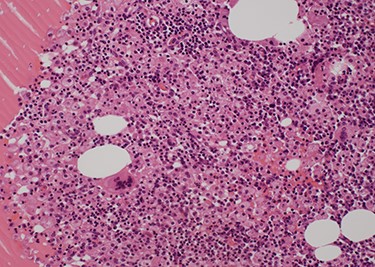
Pathological findings (Hematoxylin–Eosin staining×20). A nodular lesion with unclear boundaries is formed by fibrous cells, foamy histiocytes, foreign-body giant cells and inflammatory cell infiltration.
DISCUSSION
Xanthogranuloma is a condition that appears to be a combination of chronic inflammatory granulomatous changes with a disturbance of fat and cholesterol metabolism [6]. XA results from an unusual healing pattern of appendicitis [4]. The proposed mechanisms of the pathogenesis of XI include obstruction, such as fecaliths and fibrosis, hemorrhage, inflammation, local hypoxia, defective lipid transport, immunological disturbance, infection by low-virulence organisms, reactions to specific infectious agents and lymphatic obstruction [4, 7]; however, the conclusions are still unclear.
| Case . | Age . | Sex . | Past history . | Symptom . | Pre-operative duration . | Tumor markers . | Pre-diagnosis . | Procedure . | Post-peratively outcome . | Fecalith . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 56 | F | ND | right AP, nausea diarrhoea | 5 weeks | ND | appendix abscess with cutaneous involvement | AD | uneventful | + |
| 2 | 51 | M | multiple sclerosis | none | unknown | ND | incidentary | elective AD | ND | + |
| 3 | 66 | F | ND | right flank pain, fever | ND | ND | ND | AD | ND | ND |
| 4 | 37 | F | none | RL AP, fever | several hours | ND | acute appendicitis | AD | uneventful | ND |
| 5 | 39 | M | none | RL AP | 2 months | ND | ruptured appendicitis, diverticulitis | RHC | uneventful | ND |
| 6 | 49 | M | mitral valve disease ureteric stone | RL AP, nausea vomiting, anorexia | 13 days | ND | acute appendicitis | AD additional RHC | uneventful | ND |
| 7 | 78 | M | none | RL AP | 2 months | WNL | appendiceal mucocele | ICR | ND | ND |
| 8 | 11 | M | none | AP, emesis | 1 day | ND | acute appendicitis | laparoscopic AD | uneventful after 3 weeks | − |
| 9 | 50 | M | ND | RL AP fever, anorexia | 15 days | ND | acute inflammatory appendicular lump | RHC with ileostomy and mucous fistula | dead due to septicaemia and MOF | ND |
| 10 | 23 | F | Burkitt’s lymphoma | none | unknown | ND | chronic appendicitis the formation of a mucocele or regressd lymphoma | laparoscopic AD | uneventful | ND |
| 11 | 73 | F | none | RL AP nausea, vomiting | ND | WNL | acute appendicitis mucinous/nonmucinous EN chronic rare infectious disease | partial cecum-appendix resection hysterectomy right salpingo-oophorectomy partial bladder resection | ND | ND |
| 12 | 21 | F | ND | RF | ND | ND | acute appendicitis | AD | uneventful | − |
| 13 | 16 | M | ND | AP | 3 months | ND | recurrent acute appendicitis | interval AD | discharged on 1 day after surgery | ND |
| 14 | 30 | F | none | RF, fever | 3 weeks | ND | appendicitis | AD | ND | ND |
| 15 | 36 | M | none | RF, fever, vomiting | 1 day | ND | acute appendicitis | emergency AD | uneventful after 4 weeks | ND |
| 16 | 47 | F | ND | AP, vomiting, fever | ND | ND | inflammatory, neoplastic mass | limited right colon resection+LD | ND | ND |
| 17 | 49 | F | none | RF, fever, vomiting urinary sensations | 6 months | ND | acute on chronic appendicitis | emergency AD | uneventful after 1 month | − |
| Our case | 76 | F | AF, CI HL, HT | RL AP | 1 month | normal CEA elevated CA 19–9 | cecal cancer | Laparoscopic-assisted ICR + LD | uneventful after 20 months | − |
| Case . | Age . | Sex . | Past history . | Symptom . | Pre-operative duration . | Tumor markers . | Pre-diagnosis . | Procedure . | Post-peratively outcome . | Fecalith . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 56 | F | ND | right AP, nausea diarrhoea | 5 weeks | ND | appendix abscess with cutaneous involvement | AD | uneventful | + |
| 2 | 51 | M | multiple sclerosis | none | unknown | ND | incidentary | elective AD | ND | + |
| 3 | 66 | F | ND | right flank pain, fever | ND | ND | ND | AD | ND | ND |
| 4 | 37 | F | none | RL AP, fever | several hours | ND | acute appendicitis | AD | uneventful | ND |
| 5 | 39 | M | none | RL AP | 2 months | ND | ruptured appendicitis, diverticulitis | RHC | uneventful | ND |
| 6 | 49 | M | mitral valve disease ureteric stone | RL AP, nausea vomiting, anorexia | 13 days | ND | acute appendicitis | AD additional RHC | uneventful | ND |
| 7 | 78 | M | none | RL AP | 2 months | WNL | appendiceal mucocele | ICR | ND | ND |
| 8 | 11 | M | none | AP, emesis | 1 day | ND | acute appendicitis | laparoscopic AD | uneventful after 3 weeks | − |
| 9 | 50 | M | ND | RL AP fever, anorexia | 15 days | ND | acute inflammatory appendicular lump | RHC with ileostomy and mucous fistula | dead due to septicaemia and MOF | ND |
| 10 | 23 | F | Burkitt’s lymphoma | none | unknown | ND | chronic appendicitis the formation of a mucocele or regressd lymphoma | laparoscopic AD | uneventful | ND |
| 11 | 73 | F | none | RL AP nausea, vomiting | ND | WNL | acute appendicitis mucinous/nonmucinous EN chronic rare infectious disease | partial cecum-appendix resection hysterectomy right salpingo-oophorectomy partial bladder resection | ND | ND |
| 12 | 21 | F | ND | RF | ND | ND | acute appendicitis | AD | uneventful | − |
| 13 | 16 | M | ND | AP | 3 months | ND | recurrent acute appendicitis | interval AD | discharged on 1 day after surgery | ND |
| 14 | 30 | F | none | RF, fever | 3 weeks | ND | appendicitis | AD | ND | ND |
| 15 | 36 | M | none | RF, fever, vomiting | 1 day | ND | acute appendicitis | emergency AD | uneventful after 4 weeks | ND |
| 16 | 47 | F | ND | AP, vomiting, fever | ND | ND | inflammatory, neoplastic mass | limited right colon resection+LD | ND | ND |
| 17 | 49 | F | none | RF, fever, vomiting urinary sensations | 6 months | ND | acute on chronic appendicitis | emergency AD | uneventful after 1 month | − |
| Our case | 76 | F | AF, CI HL, HT | RL AP | 1 month | normal CEA elevated CA 19–9 | cecal cancer | Laparoscopic-assisted ICR + LD | uneventful after 20 months | − |
ND: not described, AF: atrial fibrillation, CI: cerebral infarction, HL: hyperlipidemia, HT: hypertension, RL: right lower, AP: abdominal pain, RF: right iliac fossa pain, EN: epithelial neoplasm, WNL: within normal limits, AD: appendectomy, RHC: right hemicolectomy, ICR: ileocecal resection, LD: lymphadenectomy, MOF: multiple organ failure.
| Case . | Age . | Sex . | Past history . | Symptom . | Pre-operative duration . | Tumor markers . | Pre-diagnosis . | Procedure . | Post-peratively outcome . | Fecalith . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 56 | F | ND | right AP, nausea diarrhoea | 5 weeks | ND | appendix abscess with cutaneous involvement | AD | uneventful | + |
| 2 | 51 | M | multiple sclerosis | none | unknown | ND | incidentary | elective AD | ND | + |
| 3 | 66 | F | ND | right flank pain, fever | ND | ND | ND | AD | ND | ND |
| 4 | 37 | F | none | RL AP, fever | several hours | ND | acute appendicitis | AD | uneventful | ND |
| 5 | 39 | M | none | RL AP | 2 months | ND | ruptured appendicitis, diverticulitis | RHC | uneventful | ND |
| 6 | 49 | M | mitral valve disease ureteric stone | RL AP, nausea vomiting, anorexia | 13 days | ND | acute appendicitis | AD additional RHC | uneventful | ND |
| 7 | 78 | M | none | RL AP | 2 months | WNL | appendiceal mucocele | ICR | ND | ND |
| 8 | 11 | M | none | AP, emesis | 1 day | ND | acute appendicitis | laparoscopic AD | uneventful after 3 weeks | − |
| 9 | 50 | M | ND | RL AP fever, anorexia | 15 days | ND | acute inflammatory appendicular lump | RHC with ileostomy and mucous fistula | dead due to septicaemia and MOF | ND |
| 10 | 23 | F | Burkitt’s lymphoma | none | unknown | ND | chronic appendicitis the formation of a mucocele or regressd lymphoma | laparoscopic AD | uneventful | ND |
| 11 | 73 | F | none | RL AP nausea, vomiting | ND | WNL | acute appendicitis mucinous/nonmucinous EN chronic rare infectious disease | partial cecum-appendix resection hysterectomy right salpingo-oophorectomy partial bladder resection | ND | ND |
| 12 | 21 | F | ND | RF | ND | ND | acute appendicitis | AD | uneventful | − |
| 13 | 16 | M | ND | AP | 3 months | ND | recurrent acute appendicitis | interval AD | discharged on 1 day after surgery | ND |
| 14 | 30 | F | none | RF, fever | 3 weeks | ND | appendicitis | AD | ND | ND |
| 15 | 36 | M | none | RF, fever, vomiting | 1 day | ND | acute appendicitis | emergency AD | uneventful after 4 weeks | ND |
| 16 | 47 | F | ND | AP, vomiting, fever | ND | ND | inflammatory, neoplastic mass | limited right colon resection+LD | ND | ND |
| 17 | 49 | F | none | RF, fever, vomiting urinary sensations | 6 months | ND | acute on chronic appendicitis | emergency AD | uneventful after 1 month | − |
| Our case | 76 | F | AF, CI HL, HT | RL AP | 1 month | normal CEA elevated CA 19–9 | cecal cancer | Laparoscopic-assisted ICR + LD | uneventful after 20 months | − |
| Case . | Age . | Sex . | Past history . | Symptom . | Pre-operative duration . | Tumor markers . | Pre-diagnosis . | Procedure . | Post-peratively outcome . | Fecalith . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 56 | F | ND | right AP, nausea diarrhoea | 5 weeks | ND | appendix abscess with cutaneous involvement | AD | uneventful | + |
| 2 | 51 | M | multiple sclerosis | none | unknown | ND | incidentary | elective AD | ND | + |
| 3 | 66 | F | ND | right flank pain, fever | ND | ND | ND | AD | ND | ND |
| 4 | 37 | F | none | RL AP, fever | several hours | ND | acute appendicitis | AD | uneventful | ND |
| 5 | 39 | M | none | RL AP | 2 months | ND | ruptured appendicitis, diverticulitis | RHC | uneventful | ND |
| 6 | 49 | M | mitral valve disease ureteric stone | RL AP, nausea vomiting, anorexia | 13 days | ND | acute appendicitis | AD additional RHC | uneventful | ND |
| 7 | 78 | M | none | RL AP | 2 months | WNL | appendiceal mucocele | ICR | ND | ND |
| 8 | 11 | M | none | AP, emesis | 1 day | ND | acute appendicitis | laparoscopic AD | uneventful after 3 weeks | − |
| 9 | 50 | M | ND | RL AP fever, anorexia | 15 days | ND | acute inflammatory appendicular lump | RHC with ileostomy and mucous fistula | dead due to septicaemia and MOF | ND |
| 10 | 23 | F | Burkitt’s lymphoma | none | unknown | ND | chronic appendicitis the formation of a mucocele or regressd lymphoma | laparoscopic AD | uneventful | ND |
| 11 | 73 | F | none | RL AP nausea, vomiting | ND | WNL | acute appendicitis mucinous/nonmucinous EN chronic rare infectious disease | partial cecum-appendix resection hysterectomy right salpingo-oophorectomy partial bladder resection | ND | ND |
| 12 | 21 | F | ND | RF | ND | ND | acute appendicitis | AD | uneventful | − |
| 13 | 16 | M | ND | AP | 3 months | ND | recurrent acute appendicitis | interval AD | discharged on 1 day after surgery | ND |
| 14 | 30 | F | none | RF, fever | 3 weeks | ND | appendicitis | AD | ND | ND |
| 15 | 36 | M | none | RF, fever, vomiting | 1 day | ND | acute appendicitis | emergency AD | uneventful after 4 weeks | ND |
| 16 | 47 | F | ND | AP, vomiting, fever | ND | ND | inflammatory, neoplastic mass | limited right colon resection+LD | ND | ND |
| 17 | 49 | F | none | RF, fever, vomiting urinary sensations | 6 months | ND | acute on chronic appendicitis | emergency AD | uneventful after 1 month | − |
| Our case | 76 | F | AF, CI HL, HT | RL AP | 1 month | normal CEA elevated CA 19–9 | cecal cancer | Laparoscopic-assisted ICR + LD | uneventful after 20 months | − |
ND: not described, AF: atrial fibrillation, CI: cerebral infarction, HL: hyperlipidemia, HT: hypertension, RL: right lower, AP: abdominal pain, RF: right iliac fossa pain, EN: epithelial neoplasm, WNL: within normal limits, AD: appendectomy, RHC: right hemicolectomy, ICR: ileocecal resection, LD: lymphadenectomy, MOF: multiple organ failure.
On microscopy, XI is shown to involve the recruitment of acute and chronic inflammatory cells, lipid-laden macrophages and foam cells [8]. The classic microscopic pathologic appearance of XA demonstrates numerous lipid-laden macrophages (foam cells), abundant hemosiderin and multinucleated giant cells, admixed with cholesterol clefts, and mixed inflammatory cell infiltrate [9]. The most characteristic feature is the polymorphism of cell types and the presence of foam cells filled with neutral fat, cholesterol and cholesterol esters [2, 10]. Unlike malakoplakia, no von Kossa-positive Michaelis–Gutmann bodies are observed [11].
The differential diagnosis should include the conditions showing the presence of xanthomatous cells, such as malakoplakia [8], Crohn’s disease, tuberculosis colitis and malignancy [12].
Guo et al. reported that in cases of acute appendicitis, XI was observed in 36.4% of patients who underwent delayed or interval appendectomy 4–8 weeks later and were treated with antibiotic therapy or drainage; XI was not seen in any cases of acute appendectomy [7].
XA has been reported in 17 cases in the English literature (Table 1). There were 8 men and 10 women, and the median age of these patients was 48 (range: 11–78) years. In all but two incidentally diagnosed cases, patients complained of abdominal pain. Of the three cases with tumor marker test results, only our patient exhibited elevated CA 19–9 levels. Preoperatively, no cases were diagnosed with XA, and most cases were diagnosed with typical appendicitis. Seven patients underwent not only appendectomy but also colectomy; only our patient underwent laparoscopic-assisted colectomy.
Three patients underwent surgery within 1 day of the onset. This is inconsistent with the results reported by Guo et al. A detailed medical history may have revealed similar symptoms.
Xanthogranuloma may produce yellowish tumor-like masses that appear neoplastic and can cause mechanical pressure and symptoms of obstruction [6]. In fact, some reports have noted that differentiation between XI and carcinoma in other organs is challenging [13, 14]. Because the possibility of malignancy could not be ruled out, we performed surgery.
Kahn reported that some patients with histologically typical xanthogranulomas had fatal outcomes, and the presence of anaplastic histiocytes would cause local recurrence and even metastasis [10]. Moreover, Shih noted that clinically, xanthogranuloma involving a single organ was a benign disease, whereas multi-organ involvement was a fatal disease [3]. A definitive diagnosis and curative treatment is based only on surgery [1]. Since it is not possible to determine whether the tumor is benign or malignant before and after surgery, all tumor resection should be performed as widely as possible [10]. The serum levels of CA 19–9 increase in neoplastic diseases, in non-neoplastic and organ-specific diseases, as well as in systemic diseases [15]. In the case of xanthogranulomatous cholecystitis reported by Maeda et al., the serum level of CA 19–9 increased before surgery and decreased within the normal limit after surgery. Currently, there is no literature regarding the relationship between XA and CA 19–9. It is reasonable to perform the operation for cancer and that long-term follow-up similar to that for cancer is necessary.
We believe that our case report can aid in the understanding of these rare cases and, as a result, improve their prognosis.
CONFLICT OF INTEREST STATEMENT
None declared.



