-
PDF
- Split View
-
Views
-
Cite
Cite
Ayano Sakai, Tomoyuki Okumura, Takeshi Miwa, Toru Watanabe, Yoshihisa Numata, Misato Araki, Ayaka Ito, Emi Kanaya, Taro Sakurai, Mina Fukazawa, Yui Hoshino, Yuuko Tohmatsu, Ryutaro Tokai, Hayato Baba, Katsuhisa Hirano, Takamichi Igarashi, Isaya Hashimoto, Kazuto Shibuya, Shozo Hojo, Koshi Matsui, Isaku Yoshioka, Tsutomu Fujii, Distal partial gastrectomy for gastric tube cancer with intraoperative blood flow evaluation using indocyanine green fluorescence, Journal of Surgical Case Reports, Volume 2021, Issue 12, December 2021, rjab574, https://doi.org/10.1093/jscr/rjab574
Close - Share Icon Share
Abstract
With recent advances in the treatment of esophageal cancer and long-term survival after esophagectomy, the number of gastric tube cancer (GTC) has been increasing. Total gastric tube resection with lymph node dissection is considered to be a radical treatment, but it causes high post-operative morbidity and mortality. We report an elderly patient with co-morbidities who developed pyloric obstruction due to GTC after esophagectomy with retrosternal reconstruction. The patient was treated using distal partial gastric tube resection (PGTR) and Roux-en-Y reconstruction with preservation of the right gastroepiploic artery and right gastric artery. Intraoperative blood flow visualization using indocyanine green (ICG) fluorescence demonstrated an irregular demarcation line at the distal side of the preserved gastric tube, indicating a safe surgical margin to completely remove the ischemic area. PGTR with intraoperative ICG evaluation of blood supply in the preserved gastric tube is a safe and less-invasive surgical option in patients with poor physiological condition.
INTRODUCTION
Recently, the number of gastric tube cancer (GTC) has increased [1]. Although total gastric tube resection (TGTR) is considered as a radical treatment, it is invasive with a high post-operative morbidity and mortality [2–4]. We report an elderly patient with GTC who was treated using partial gastric tube resection (PGTR) and Roux-en-Y reconstruction with preservation of the right gastroepiploic artery (RGEA) and right gastric artery (RGA). Intraoperative visualization of the blood flow in the preserved gastric tube using indocyanine green (ICG) fluorescence was useful to decide the proximal surgical margin.
CASE REPORT
An 80-year-old man developed gastric tube adenocarcinoma after radical esophagectomy for squamous cell carcinoma. He underwent video-assisted subtotal esophagectomy followed by reconstruction of the gastric tube through the retrosternal route and cervical anastomosis 7 years ago. Based on his age and co-morbidities, such as interstitial pneumonia, paroxysmal atrial fibrillation, aortic regurgitation and hypertension, he has been followed up by regular check-ups without adjuvant therapy.
Upper gastrointestinal endoscopy at the seventh year of follow-up revealed a polypoid tumor of 3 cm in diameter at the pyloric region of the gastric tube with pyloric obstruction (Fig. 1). Endoscopic biopsy from the tumor revealed papillary adenocarcinoma (pap > tub1). Contrast-enhanced computed tomography (CT) demonstrated a solid mass with slight enhancement at the abdominal part of the gastric tube located below the lower border of the sternum (Fig. 2). Neither lymph node metastasis nor distant metastasis was detected, leading to a diagnosis of clinical stage IB (cT2N0M0) gastric cancer according to the seventh edition of the Union for International Cancer Control system.
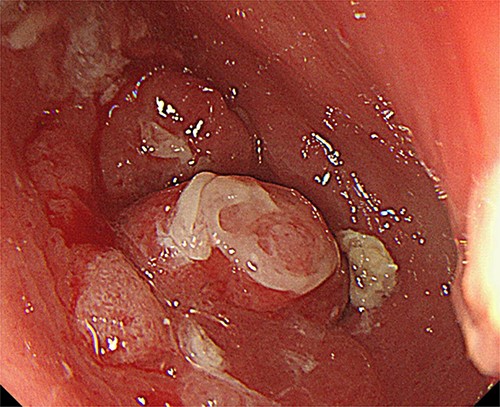
Upper gastrointestinal endoscopy showed a tumor at the pyloric region of the gastric tube causing pyloric obstruction.
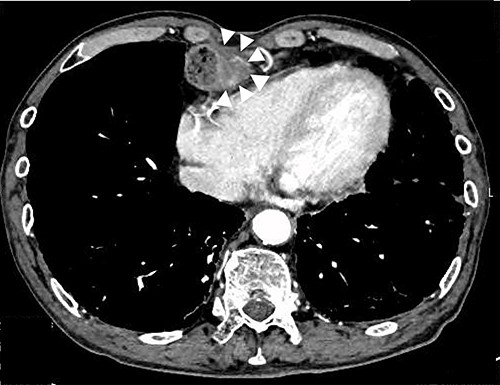
Preoperative CT image; white arrows indicate a tumor with thickening of the gastric wall.
TGTR with sternotomy was considered a radical surgery; however, in consideration of surgical invasion and the patient’s physical condition, gastric vessel-preserving partial distal gastrectomy was conducted.
Under upper midline laparotomy, the abdominal part of the gastric tube was mobilized along with the RGEA and RGA after careful dissection. The first and second branches of the RGEA and RGA were divided along the gastric wall, carefully preserving the main vessels. Supra-duodenal arteries were also divided. Then, the anal side margin was set at 1 cm from the distal side of the pylorus, and the duodenum was divided using a linear stapler (Fig. 3A). Before setting the proximal surgical margin, blood supply in the gastric tube was assessed by ICG fluorescence [5]. The visualized ICG fluorescence demonstrated an irregular demarcation line at the distal side of the preserved gastric tube, indicating insufficient blood supply at the greater and lesser curvature compared with the center of the gastric wall (Fig. 4). The gastric tube was thus divided at 2 cm on the oral side from the proximal margin of the tumor, confirming complete removal of the ischemic area. Then gastro-jejunostomy was performed for Roux-en-Y reconstruction (Fig. 3B).
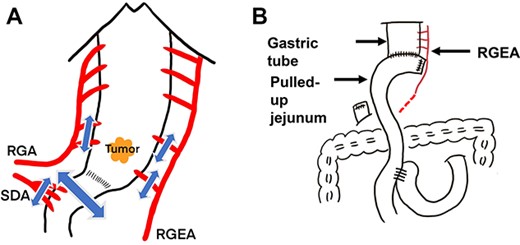
Diagrammatic representation of the intraoperative findings; (A) resection of the distal gastric tube with preservation of the RGEA and RGA and (B) Roux-en-Y reconstruction; SDA, supra-duodenal artery; p-ring, pyloric ring.
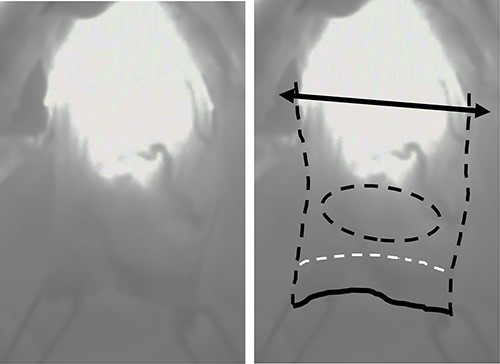
Intraoperative visualization of the blood flow in the proximal side of the gastric tube using ICG fluorescence; bilateral arrow: dissection line of the proximal margin; black dotted line: outline of the proximal part of the gastric tube; black solid line: distal surgical margin; white dotted line: pylorus; black dotted circle: location of the tumor.
The surgery was uneventful. The operation time was 3 h 49 min, and the amount of blood loss was 60 g. The patient fully recovered and was discharged 16 days after surgery.
The resected specimens are shown in Fig. 5. Pathological examination of the surgical specimen demonstrated moderately differentiated adenocarcinoma (pT2), indicating R0 resection. The patient is healthy at 26 months after surgery without recurrence.
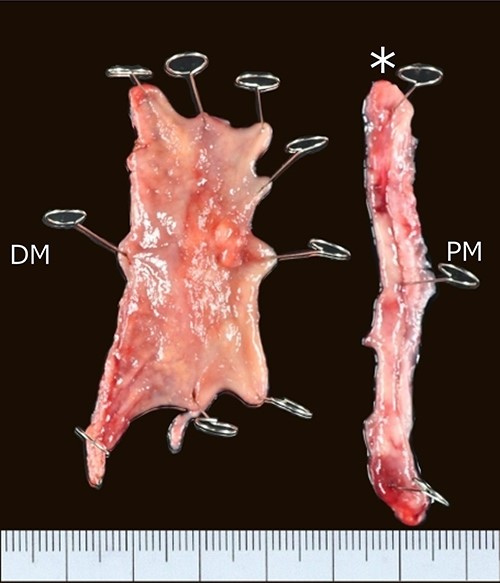
Resected specimen; a Type II tumor in the pyloric lesion of the gastric tube measuring 25 × 15 mm in size; PM, proximal margin; DM, distal margin; asterisk, surgical specimen removed by circular staple.
DISCUSSION
Recent progress in surgical techniques and the development of novel therapeutic modalities, such as adjuvant chemoradiation therapy combined with surgery, have improved the post-operative survival up to 34–51% at 5 years [1]. In addition, the mean interval between esophagectomy and diagnosis of GTC was reported to be 55.8 months [6], suggesting that more elderly patients will develop GTC in the future.
If endoscopic submucosal resection is not indicated, most patients with resectable GTC will receive TGTR or PGTR [6]. TGTR with lymph node dissection is considered as a radical treatment for GTC. In many cases, distal para-gastric lymph node stations No. 5, No. 6 and No. 4d [7], which were not removed during prior esophagectomy, are dissected. Then, TGTR is completed with reconstruction using colon or jejunal interposition [8]. However, the extent of lymphadenectomy in GTC has not been standardized because of the lack of long-term surgical outcomes [9]. In addition, surgical removal of the reconstructed gastric tube requiring sternotomy or thoracotomy depends on the reconstruction route, is invasive and involves a high risk of post-operative morbidity and mortality [2–4], especially for elderly patients [10]. On the other hand, because the majority of GTC cases are located in the lower part of the gastric tube, PGTR is also performed as a radical treatment in patients with early stage GTC for which endoscopic resection is difficult because of the location, such as on the staple line or pyloric ring [6, 8, 10]. In the present case, PGTR without radical lymph node dissection, preserving the RGEA and RGA, was performed as a palliative surgery for an 80-year-old patient with clinical stage IB GTC.
On the intraoperative evaluation of blood flow in the gastric tube after clamping or ligation of the RGEA and RGA, several reports noted neo-vascularization from the remnant cervical esophagus to the upper region of the gastric tube. However, the length of the vascularized gastric tube varies among patients [4, 9, 11–13]. On the other hand, the length of the proximal gastric tube with sufficient blood flow was 3–7 cm from the esophago-gastric anastomosis in other reports [4, 11, 12], suggesting that TGTR will be needed in the majority of cases after dissection of the RGEA and RGA.
We performed intraoperative ICG evaluation to detect the blood supply in the preserved gastric tube. Recent reports suggested the use of blood supply visualization using ICG methods allows preservation of the proximal portion of the gastric tube after dissection of the RGEA and RGA [4, 11, 12]. In the present case, even during PGTR with preservation of the RGEA and RGA, intraoperative detection of ICG fluorescence was useful to safely set the proximal surgical margin of the gastric tube based on the sufficient blood supply at the anastomotic lesion.
In conclusion, PGTR with intraoperative ICG evaluation of blood supply in the preserved gastric tube is a safe and less-invasive surgical option in patients with a poor physiological condition.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to declare.
FUNDING
This work was partly supported by a Grant-in-Aid for Scientific Research (C) MEXT KAKENHI Grant Number 18K08642. None of the authors have financial competing interests to disclose.
References
- ischemia
- vascular flow
- cancer
- roux-en-y anastomosis
- esophagectomy
- fluorescence
- gastroepiploic artery
- indocyanine green
- intraoperative care
- reconstructive surgical procedures
- surgical procedures, operative
- pyloric stenosis
- morbidity
- mortality
- lymph node dissection
- older adult
- gastric bypass, roux-en-y
- subtotal gastrectomy
- right gastric artery
- surgical margins
- gastric tube reconstruction



