-
PDF
- Split View
-
Views
-
Cite
Cite
Paolo Gasparella, Georg Singer, Christoph Castellani, Erich Sorantin, Emir Q Haxhija, Holger Till, Giant lymphatic malformation causing abdominal compartment syndrome in a neonate: a rare surgical emergency, Journal of Surgical Case Reports, Volume 2020, Issue 8, August 2020, rjaa252, https://doi.org/10.1093/jscr/rjaa252
Close - Share Icon Share
Abstract
Abdominal lymphatic malformations in neonates require sophisticated management. In symptomatic cases, acute complications may necessitate immediate surgery. We present the case of a giant abdominal lymphatic malformation diagnosed in the 18th gestational week. Sonographic concerns about intestinal hypoperfusion in the 33rd week of gestation indicated caesarean section. Postnatal imaging confirmed a macrocystic lymphatic malformation occupying almost the complete abdominal cavity; the intestinal perfusion was normal. Clinical deterioration on Day 13 of life required laparotomy. Intraoperatively, the lymphatic mass was located in the ileocecal mesentery. Two major cysts showed recent hemorrhage explaining the onset of abdominal compartment syndrome. The malformation was completely removed. An ileocecal resection with an ileocolic anastomosis was performed. The postoperative course was uneventful. In neonates with abdominal lymphatic malformations, an onset of abdominal compartment syndrome requires surgical exploration. If feasible, the complete removal of the lesion represents a curative option.
INTRODUCTION
Lymphatic malformations (LMs) are congenital lesions consisting of dilated lymphatic channels lined by lymphatic endothelial cells [1]. Due to their infiltrative nature, it remains difficult to localize the borders of the lesions even with the most accurate diagnostic tools. LMs are rarely localized intra-abdominally. In these cases, a differentiation from other cystic lesions may be possible already prenatally. However, a statement about their exact origin and extension is particularly challenging [2]. The optimal treatment regimen for large abdominal LMs has not been defined yet. An explorative laparotomy could lead to catastrophic consequences like extensive bowel resections, whereas sclerotherapy could expose the patients to life-threatening complications [3].
We report the case of the prenatally diagnosed giant abdominal LM treated surgically in the neonatal period due to an abdominal compartment syndrome.
CASE REPORT
In the 18th week of gestation, routine ultrasound examination of a 35-year-old primigravida revealed a fetal cystic intra-abdominal mass occupying the entire abdominal cavity. A prenatal magnetic resonance imaging (MRI), performed in the 29th gestational week, showed a lymphatic cystic malformation measuring 12 × 7 × 9 cm (Fig. 1). In the 33rd gestational week, intestinal hypoperfusion due to compression by the malformation was suspected during an ultrasound examination. Therefore, a caesarean section was performed without complications.
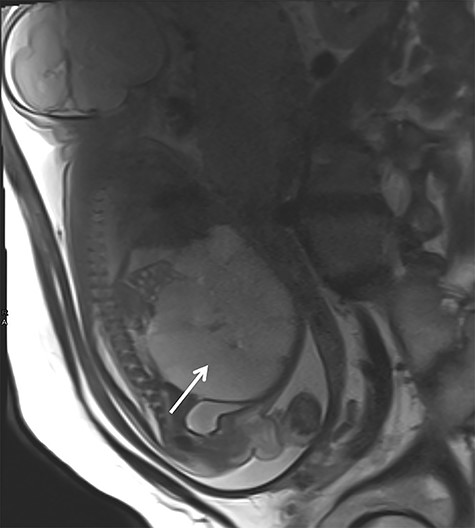
Prenatal MRI in the 29th gestational week showing a multicystic septed mass occupying the complete abdominal cavity (white arrow).
After birth, the male baby presented in good general condition with stable vital signs. The abdomen was distended but not painful on palpation. Postnatal ultrasound confirmed the lesion. To assess the exact extend, MRI was performed showing a macrocystic LM occupying the entire abdomen (Fig. 2) without ascites. However, the exact origin of the lesion and the amount of extension into the mesenteric root could not be described.
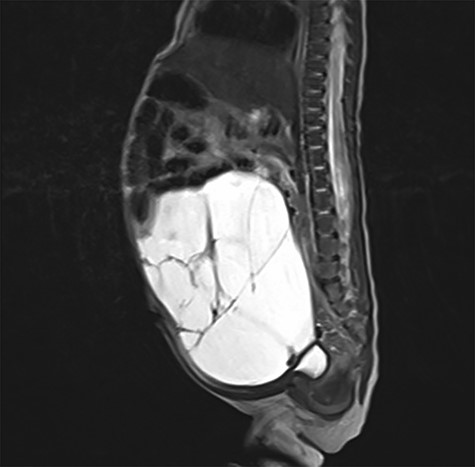
Postnatal abdominal MRI. The bowel loops are displaced into the upper part of the abdomen. The exact origin of the lymphatic malformation could not be described.
On the 13th day of life, the boy suddenly developed massive abdominal distension and compartment syndrome requiring tracheal intubation because of respiratory distress. Hemoglobin dropped from 15.7 to 9.6 g/dl within 3 hours and lactate was elevated with 8.8 mmol/l. Emergency laparotomy was performed for decompression and demonstrated that the cystic mass occupied the ileocecal mesentery. Two major cysts were hemorrhagic explaining the abdominal compartment syndrome (Fig. 3a). An extended ileocecal resection allowed the complete removal of the LM. Subsequently, a termino-terminal ileocolic anastomosis was created (Fig. 3b).
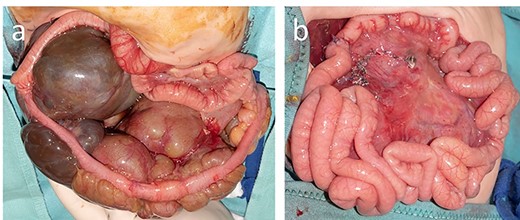
At laparotomy, the lymphatic malformation was limited to the ileocecal mesentery (a), and after complete removal, it was possible to perform an ileocolic termino-terminal anastomosis (b).
Histological examination confirmed the diagnosis of a cystic LM with acute hemorrhage.
The postoperative course was uneventful. Abdominal ultrasounds were performed 1, 3, 6, 12 and 18 months postoperatively and were without evidence of recurrence.
DISCUSSION
The lymphatic system develops in the fifth gestational week from an endothelial sprouting outgrowth of the venous system. Alterations at this developmental stage cause the formation of dysfunctioning lymphatic collectors termed LMs. Depending on the size of the cysts, the International Society for the Study of Vascular Anomalies distinguishes macrocystic and microcystic subtypes [1].
In 75% of the cases, LMs are localized in the head–neck region and about 20% develop in the axillary–chest wall region [4, 5]. Abdominal LMs are rare and constitute about 5% of all LMs [5, 6]. In most cases, they are found in the mesentery [7].
LMs normally are diagnosed either prenatally during routine sonographic screening examinations or postnatally when complications occur. Fetal MRI has acquired a fundamental role in order to differentiate abdominal LMs from other lesions such as choledochal cysts, renal pelvis dilatations and intestinal duplications. In our patient, MRI confirmed the diagnosis of an abdominal LM suspected with sonography. During a subsequent control ultrasound examination, intestinal ischemia was suspected necessitating preterm delivery in the 33th gestational week. To the best of our knowledge, similar cases have not been reported in the recent literature.
Abdominal LMs are often diagnosed immediately after birth or during infancy, usually when they become symptomatic [2]. The most frequently reported onset symptom is abdominal pain, which often presents acutely due to severe complications such as volvulus, infection, rupture or intralesional hemorrhage [5]. In the case of voluminous lesions like in our patient, intracystic bleeding can cause a sudden increase in the size of the malformation associated with a dangerous increase in intra-abdominal pressure. If the patient’s condition permits it, further diagnostic procedures like MRI, which allow a better definition, should be performed [5]. The peculiar difficulty of the present case consisted in the lack of diagnostic definition of the malformation’s borders. Nevertheless, we were forced to perform an explorative laparotomy because of the rapid worsening of the patient’s clinical condition. At laparotomy, it became evident that intracystic bleeding was the cause of the abdominal compartment syndrome.
Surgical excision is considered the first-choice treatment of abdominal LMs only if it is not mutilating and can be performed completely. Unfortunately, despite the fact that LMs are benign lesions, they usually invade neighboring structures, making a radical excision challenging. Furthermore, high recurrence rates in cases of incomplete excision have been reported. The two possible surgical options are either the removal of the cyst alone or the removal of the cyst with the infiltrated structures [5].
For unresectable abdominal LM, sclerotherapy consisting of the local injection of different agents such as picibanil (OK-432), bleomycin or doxycycline has been shown to be a therapeutic option [7, 8]. However, complications like abdominal compartment syndrome as well as the possible damage of the communicating lymphatic channels resulting in abdominal lymphatic congestion are possible [3, 8]. In our patient, before the condition of the patient suddenly worsened, the lack of a definitive diagnosis and the concern to cause an abdominal compartment syndrome made us hesitant to perform sclerotherapy. Recently, the use of rapamycin for treating LMs has been reported as feasible and successful in reducing the volume of lymphatic lesions [9].
In conclusion, in cases of prenatally diagnosed abdominal LMs, it is essential to plan an accurate postnatal diagnostic procedure as well as the appropriate treatment approach. After diagnosis, treatment is indicated because of possible severe complications, which can develop suddenly and become life-threatening. If feasible, complete surgical removal should be the goal, as it will minimize the rate of recurrence.
Conflict of interest statement
The authors declare that they have no relevant or material financial interests that relate to the research described in this manuscript.
Funding
None.



