-
PDF
- Split View
-
Views
-
Cite
Cite
Emily Ann Harris, Rafael Garza Castillon, Priya Bhakta, Do hepatic artery infusion pumps cause recurrent pleural effusions?, Journal of Surgical Case Reports, Volume 2020, Issue 8, August 2020, rjaa137, https://doi.org/10.1093/jscr/rjaa137
Close - Share Icon Share
Abstract
Hepatic artery infusion pump chemotherapy (HAIPC) for colorectal liver metastasis (CRLM) is a new technique in the treatment of CRLM, whose side effects are not well studied. Case Report: This paper aims to understand the side effect profile of HAIPC as it relates to recurrent pleural effusions. This is a case report of a 48-year-old male with CRLM being treated with HAIPC, who presents with recurrent pleural effusions found to be benign/transudative after right-side video-assisted thoracoscopic surgery. Discussion: This study suggests that HAIPC causes recurrent sympathetic pleural effusions as a side effect of the perihepatic inflammation of the localized chemotherapy treatment. Furthermore, we question if sympathetic pleural effusions are a prelude to hepatic toxicity from HAIPC. Lastly, this paper aims to guide the differential diagnosis of pleural effusions in the cancer patient being treated with HAIPC.
INTRODUCTION
Hepatic artery infusion pump chemotherapy (HAIPC) is a new technology used in patients with colorectal liver metastases (CRLMs), but it remains infrequently used [1] with a limited understanding of its side effects. Here, we present a case report of a benign recurrent right pleural effusion in a 48-year-old patient with CRLM and HAIPC. This paper suggests that recurrent pleural effusions are a side effect of HAIPC in the treatment of CRLM.
The liver is the most common site of metastasis of colorectal cancer with up to 60% of patients developing liver metastases during the course of their disease [1–3]. In colorectal cancer patients with liver dominant metastatic disease, HAIPC combined with systemic agents demonstrates an improved liver tumor response compared with systemic therapy alone (43 vs 18%) [2]. This success is owed to the fact that CRLMs receive their blood supply predominately from the hepatic artery, and therefore, HAIPC provides high intra-tumoral chemotherapy concentration [3]. We hypothesize that the localized toxicity and inflammatory effect from the HAIPC stimulates a sympathetic pleural effusion due to a sub-clinical hepatitis.
CASE REPORT
A 48-year-old male with a history of metastatic rectal cancer to the liver status post-folfox and bevacizumab, proctosigmoidectomy, coloanal anastomosis, hepatectomy and hepatic artery infusion pump placement (May 2019), who on December 2019 presented to thoracic surgery with a recurrent right pleural effusion (Fig. 1). Of note, the patient was asymptomatic from this effusion (Fig. 1). The patient’s last infusion using the HAIPC occurred on November 2019.
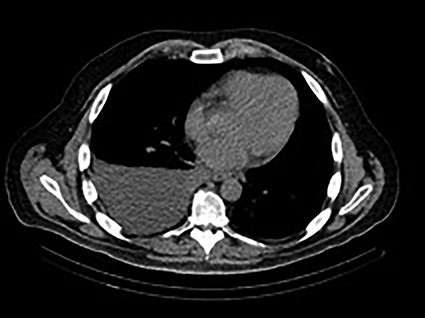
December 2019—CT chest: moderate to large right-sided pleural effusion.
Since the placement of his HAIPC, the patient had two right-sided thoracentesis demonstrating transudate fluid (Table 1). The patient had multiple interrogations of his HAIP including a Nuclear Medicine Hepatic Pump w/SPECT/CT (Fig. 2) and an IR Venogram (Fig. 3), demonstrating adequate position and functioning of the HAIP with perfusion of the liver and no extrahepatic radiotracer. Of note, the patient’s labs pre-operatively were all within normal limits, including normal biliary labs (AST/ALT 23/15, AP 55 and T bili .3).
| . | Triglycerides mg/dl . | Protein g/dl . | pH . | LDH Unit/l . | Albumin g/dl . | Glucose mg/dl . | Aerobic w/gram . | Acid fast bacili . |
|---|---|---|---|---|---|---|---|---|
| September 2019 | 42 | 4.9 | 7.40 | 127 | 3.1 | 119 | Negative | Negative |
| November 2019 | 51 | 4.6 | 7.61 | 100 | 2.7 | 97 | Negative | Negative |
| December 2019 | 55 | 4.5 | 7.50 | 116 | 2.9 | 111 | Negative, rare wbc | Negative |
| . | Triglycerides mg/dl . | Protein g/dl . | pH . | LDH Unit/l . | Albumin g/dl . | Glucose mg/dl . | Aerobic w/gram . | Acid fast bacili . |
|---|---|---|---|---|---|---|---|---|
| September 2019 | 42 | 4.9 | 7.40 | 127 | 3.1 | 119 | Negative | Negative |
| November 2019 | 51 | 4.6 | 7.61 | 100 | 2.7 | 97 | Negative | Negative |
| December 2019 | 55 | 4.5 | 7.50 | 116 | 2.9 | 111 | Negative, rare wbc | Negative |
| . | Triglycerides mg/dl . | Protein g/dl . | pH . | LDH Unit/l . | Albumin g/dl . | Glucose mg/dl . | Aerobic w/gram . | Acid fast bacili . |
|---|---|---|---|---|---|---|---|---|
| September 2019 | 42 | 4.9 | 7.40 | 127 | 3.1 | 119 | Negative | Negative |
| November 2019 | 51 | 4.6 | 7.61 | 100 | 2.7 | 97 | Negative | Negative |
| December 2019 | 55 | 4.5 | 7.50 | 116 | 2.9 | 111 | Negative, rare wbc | Negative |
| . | Triglycerides mg/dl . | Protein g/dl . | pH . | LDH Unit/l . | Albumin g/dl . | Glucose mg/dl . | Aerobic w/gram . | Acid fast bacili . |
|---|---|---|---|---|---|---|---|---|
| September 2019 | 42 | 4.9 | 7.40 | 127 | 3.1 | 119 | Negative | Negative |
| November 2019 | 51 | 4.6 | 7.61 | 100 | 2.7 | 97 | Negative | Negative |
| December 2019 | 55 | 4.5 | 7.50 | 116 | 2.9 | 111 | Negative, rare wbc | Negative |
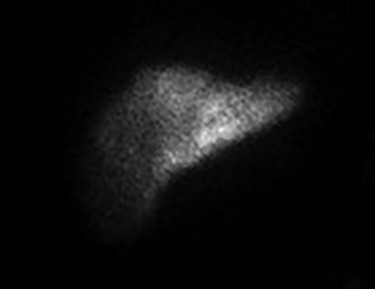
December 2019—IR procedure: NUC MED HEPATIC PUMP with SPECT/CT—appropriate perfusion of the liver after injection of radiotracer into the hepatic arterial infusion pump. No evidence of extrahepatic radiotracer collection identified.
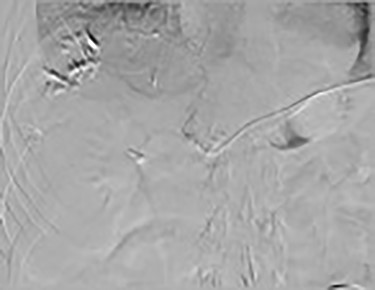
December 2019: procedure—IR venogram PROCEDURE: Hepatic Arterial Infusion Pump Evaluation, MAA Infusion—an angiogram was performed via the pump showing opacification of both right- and left-side hepatic arteries.
On 20 December 2019, the patient underwent a right-side video-assisted thoracoscopic surgery, with pleural biopsy, fluid cytologic analysis and pleurex catheter placement. In surgery, 600 cc of amber clear pleural fluid was drained; several tan areas on the parietal pleura were biopsied and sent for both frozen and permanent pathologic analysis. Frozen pathology was negative for malignancy, and final pathology demonstrated right pleural biopsy with markedly reactive mesothelial cells with chronic and minimal acute inflammation. The post-operative pleural fluid analysis was transudative (Table 1, December 2019).
DISCUSSION
This report of a 48-year-old male with history of CRLM being treated with HAIPC serves as the sentinel paper documenting the association of chronic pleural effusions with HAIPC. Of note, this is a diagnosis of exclusion.
In regard to HAIPC-related adverse events, pleural effusions are not documented as a side effect. Rather, the most common toxicities were diarrhea, neutropenia and neurotoxicity [2]; other problems were related to hepatobiliary and upper gastrointestinal inflammation [3]. HAIPC catheter-related complications were 22% and include: arterial thrombosis (6%), occlusion/dislodgement (6%), extrahepatic perfusion (3%), site infection/hematoma (3%) and biliary sclerosis (5%) [2, 3].
This paper suggests that the patient’s pleural effusion is due to sympathetic hepatic inflammation from the local regional chemotoxicity of the HAIPC. This conclusion is reinforced by the fact that chemically induced hepatitis is common with HAIPC ranging from 30 to 70% [3]. Although our patient did have normal biliary labs, a subclinical hepatitis cannot be excluded as the metastatic liver tissue receiving the toxic therapy that has minimal functioning tissue to manifest a hepatitis in laboratory evaluation. Therefore, LFTs can be normal in patients with chronic liver disease, as evidenced by at least one-third of patients with chronic hepatitis having normal serum ALT levels despite the presence of inflammation on liver biopsy [4]. This local regional toxicity of the metastatic liver, which spares the healthy liver remnant, is reinforced by the fact that liver metastases are perfused almost exclusively by the hepatic artery, and normal liver tissue receives its blood supply from mainly portal circulation [1].
The most limiting hepatic toxicity related to HAIPC is biliary sclerosis [5], which manifests by elevations in serum alkaline phosphatase, AST/ALT and bilirubin [2, 6], and occurs in approximately 1–26% of patients [3]. It has been reported that the use of systemic bevacizumab in conjunction with HAI–FUDR significantly increases the risk of biliary sclerosis [3]. Of note, our patient in this case report received bevacizumab from October 2018–March 2019.
One question concerns whether this sub-clinical hepatitis in our case report is a risk factor for the development of primary biliary cirrhosis PBC. PBC is a chronic inflammatory cholestatic disease resulting from genetic and environmental triggers, which results in sclerosis of small intrahepatic ducts causing elevations in AP, AST and ALT that are in proportion to the severity of the disease [6]. However, in stage 1 PBC, there is minimal portal inflammation without florid bile duct lesions [6]; therefore, it could be argued that in early stages of PBC, there may not be biochemical markers to suggest its presence. Although our patient in question had normal levels of serum AP, AST/ALT and bilirubin, he did demonstrate evidence of hepatic inflammation as evidenced by his sympathetic right pleural effusion, and therefore, a liver biopsy may be warranted to rule out this pathology. Lastly, in patients with CRLMs being treated with HAIPC, it is critical to differentiate between a benign and malignant pleural effusion, as HAIPC is not recommended in the setting of extrahepatic disease [1] and therefore influences treatment.
Therefore, recurrent pleural effusions in a patient with HAIPC for CRLM can be a side effect of the regionally directed HAIPC (rather than progression of the malignancy) and may warrant a pleurex catheter placement if the patient is to have continued HAIPC infusions. Furthermore, sub-clinical hepatitis as evidenced by sympathetic pleural effusions should make the clinician aware of possible increased risk factor for the development of primary biliary sclerosis in this patient population and may warrant a liver biopsy for diagnosis.
Conflict of Interest
None declared.
Funding
None.



