-
PDF
- Split View
-
Views
-
Cite
Cite
Johan O Wedin, Mikael Janiec, Petter Schiller, Commando procedure in a patient with double-valve endocarditis after transcatheter aortic valve implantation, Journal of Surgical Case Reports, Volume 2020, Issue 8, August 2020, rjaa079, https://doi.org/10.1093/jscr/rjaa079
Close - Share Icon Share
ABSTRACT
The indication for transcatheter aortic valve implantation (TAVI) is continuously expanding with a simultaneous increase in number of TAVI associated prosthetic valve endocarditis (TAVI–PVE). Evidence for management of TAVI–PVE is lacking but the need for surgical management of complex TAVI–PVE is expected to increase. The Commando procedure, a technically challenging surgery for treatment of complex endocarditis, has never been described in a patient with TAVI–PVE. An 80-year-old female with TAVI–PVE, native mitral valve endocarditis and an abscess in the intervalvular fibrous body was admitted to our clinic. She successfully underwent the Commando procedure with implantation of biological mitral and aortic valve prostheses and reconstruction of the intervalvular fibrous body. We demonstrate that the Commando procedure is a feasible surgical alternative in patients with TAVI–PVE and that it can be considered in patients with high surgical risk.
INTRODUCTION
Prosthetic valve endocarditis (PVE) is associated with high mortality rates, why an early surgical strategy is recommended [1]. The indication for Transcatheter Aortic Valve Implantation (TAVI) is expanding to low-risk patients [2] and the need for surgical management of complex TAVI–PVE is expected to increase. The Commando procedure is a technically challenging procedure where the aortic and mitral valves (either native or prosthetic valves) are replaced, and the intervalvular fibrous body is reconstructed [3]. This surgical approach has never been described in TAVI–PVE.
CASE REPORT
An 80-year-old woman with a medical history of hypertension and obesity developed exertional dyspnea in late 2018. An echocardiogram showed a severe aortic stenosis while the coronary angiogram was unremarkable. The Heart Team decided in favor for TAVI and in April 2019, a Medtronic Evolut R 26 mm was implanted. The postoperative course was uneventful. The routine control at the cardiologist 1 month after the TAVI procedure showed satisfying results with an improved performance on the 6-min walk test, and an unremarkable physiological examination.
In October 2019, she was referred from the primary care physician due to a 3-week history of cough and fever chills and a C-reactive protein (CRP) of 128 mg/L (reference < 5 mg/L). A systolic murmur raised suspicion of infective endocarditis. The diagnosis was confirmed with transesophageal echocardiography (TEE), which in addition to mitral and prosthetic valve vegetations of up to 20 mm, also showed an abscess in the intervalvular fibrous body (Fig. 1). Blood cultures revealed growth of Cutibacterium spp. After a thorough discussion with the patient, the decision for surgery was taken.
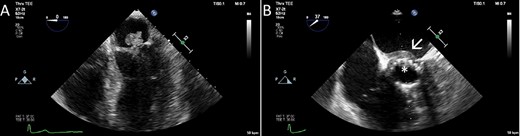
The TEE showed a large vegetation on the native mitral valve (A), vegetations on the TAVI prosthesis (B, asterisk) and an abscess in the intervalvular fibrous body (B, arrow).
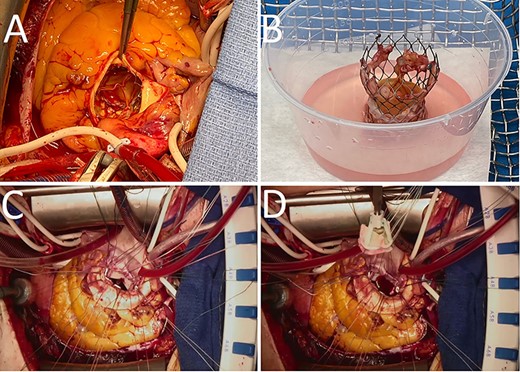
Gross images of the TAVI prosthesis showing vegetations in the stent ((A), (B)). The biological mitral (C) and aortic valve (D) prostheses were attached to a bovine pericardial patch, which was used to reconstruct the intervalvular fibrous body.
A full-median sternotomy was performed, and the heart was accessed after opening of the pericardial sac. After bicaval and aortic arch cannulation, the patient was put on cardio-pulmonary bypass and antegrade cardioplegia was delivered to induce asystole. A combination of antegrade and retrograde cardioplegia was iterated every 20 min during the procedure. The ascending aorta was incised above the TAVR prosthesis (Fig. 2A), on which small vegetations were found on the stent as well as on the leaflets (Fig. 2B). The TAVR prosthesis was removed and after removal of the native aortic valve, an abscess was found at the level of the commissure between the left- and non-coronary cusps. The aortotomy was therefore extended toward this area for better exposure, and the abscess had almost penetrated into the left atrium. The superior vena cava was opened above the sinus node to allow a better exposure to the left atrium. The left atrium was subsequently opened in the roof and the incision was connected to the aortotomy and further down to the anterior mitral leaflet which was excised. Pledgeted sutures were placed in the posterior mitral anulus and sutured to the posterior two-thirds of a 27 mm Perimount Magna, while the anterior one-third of the mitral valve prosthesis was attached to a bovine pericardial patch (Fig. 2C). A 21 mm Perimount Magna aortic valve prosthesis was then attached to the aortic anulus and the rest of the bovine pericardial patch with pledgeted sutures (Fig. 2D). The left atriotomy was closed using one side of the pericardial patch, and the other side of the pericardial patch was sutured to close the aortic root before continuing with the closure of the aortotomy. Perioperative TEE showed well-functioning biological prosthesis in both mitral and aortic positions without signs of paravalvular leak, and there was no flow across the bovine pericardial patch. The weaning from cardio-pulmonary bypass was relatively uncomplicated although the patient developed a third-degree atrioventricular block without junctional escape rhythm. Epicardial pacemaker electrodes were sutured to the right atrium and right ventricle and tunneled to the left infraclavicular fossa.
After spending 3 days on the thoracic intensive care unit, the patient was transferred to the surgical ward to continue intravenous antibiotic therapy. The postoperative course was uneventful and on the seventh postoperative day, a pacemaker generator was connected to the tunneled electrodes. Routine postoperative echocardiogram confirmed the perioperative findings with well-functioning biological prostheses in aortic and mitral positions. Postoperative chest X-ray also showed ordinary findings (Fig. 3). Cultures from the native mitral valve and the TAVR prosthesis were positive for Cutibacterium spp. Initially, the patient was treated with a combination of Vancomycin and Cefotaxime. Cefotaxime was withdrawn after 2 weeks and Vancomycin in single therapy was continued another 2 weeks, and the patient was discharged to her home after 4 weeks of postoperative intravenous antibiotic therapy. At discharge, she was feeling well with no fever, a CRP concentration of 13 mg/L and a leukocyte count of 5.0 x 109/L (reference 3.5–9.0 x 109/L).
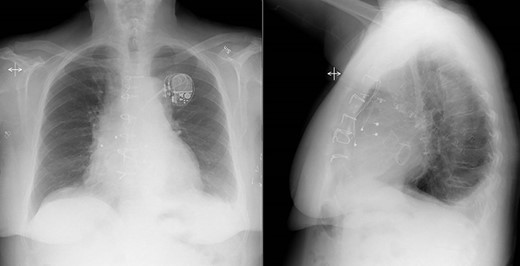
Postoperative chest X-ray showed ordinary findings with only a small amount of bilateral pleural effusion and adjacent pulmonary atelectasis.
DISCUSSION
We report a complicated case of PVE in an elderly TAVR patient, where also the native mitral valve and the intervalvular fibrous body was infected. The patient underwent replacement of both infected valves as well as reconstruction of the intervalvular fibrous body, known as the Commando procedure. This has never been described in a patient with TAVR–PVE.
The incidence of PVE is similar in patients that undergo surgical aortic valve replacement and TAVI, occurring in 0.5–1.5% per year. The outcome is poor in both groups but appears to be worse for TAVI–PVE [4]. When optimal medical therapy is failing, or in the case of an intervalvular abscess, surgical replacement of an infected valve prosthesis is recommended. The existing data on outcome in TAVI–PVE is derived from retrospective studies including a limited number of patients with short follow-up and therefore no general recommendations for management of TAVI–PVE exist. With expanding indications for TAVI and a rapidly growing number of TAVI procedures, the frequency of TAVI–PVE is expected to increase, with an increasing number of patients being surgical candidates. The decision on appropriate therapy should be performed on a case-to-case basis. This case illustrates that the Commando procedure, with replacement of a TAVI prosthesis, a native mitral valve and reconstruction of the intervalvular fibrous body, is a feasible surgical alternative in patients presenting with TAVI–PVE.



