-
PDF
- Split View
-
Views
-
Cite
Cite
Quinlan Carlson, Umamaheshwari Golconda, Belinda Sun, Gastric heterotopia with perforation mimicking neoplastic process in ileum, Journal of Surgical Case Reports, Volume 2020, Issue 7, July 2020, rjaa224, https://doi.org/10.1093/jscr/rjaa224
Close - Share Icon Share
Abstract
Gastric heterotopia (GH) is rare in ileum except in Meckel’s diverticulum and rarely causes severe symptoms in adults. Here, we report a 31-year-old male patient with GH in ileum presented with bowel perforation and mass formation in the mesentery mimicking perforated small bowel tumor. Microscopic examination of the lesion showed completely differentiated gastric body-type mucosa with mucosal ulceration, fistula formation and bowel perforation. This case raises the awareness that GH may cause severe complications and should be included in the differential diagnosis for acute abdominal pain especially in patients with a mass lesion at an unusual location.
INTRODUCTION
Gastric heterotopia (GH) is defined as the presence of gastric tissue type at a nonphysiological site [1]. Although it has been reported in almost every anatomical site in the gastrointestinal (GI), GH is commonly seen in esophagus and proximal duodenum, much less beyond the ligament of Treitz, and rarely encountered in ileum except for its presence in Meckel’s diverticulum [1, 2]. GH is often an incidental endoscopic or microscopic finding and usually asymptomatic. Even symptomatic, GH usually causes mild GI symptoms such as abdominal pain or melana [1]. However, here, we report a case of GH in ileum presented with bowel perforation mimicking tumor in a 31-year-old male who underwent exploratory laparotomy and small bowel resection.
CASE REPORT
Clinical history: a 31-year-old male without significant past medical history presented with abdominal pain for 2 days. He initially visited an outside hospital emergency department for abdominal pain and was diagnosed as ‘enteritis’ treated with ciprofloxacin and metronidazole and discharged home. His pain did not improve so he returned to the emergency department the next day. Abdominal computed tomography scan showed dilated bowel and free air, as well as a 2.4 × 2.0 cm unclear lesion in the right lower quadrant. The patient was transferred to the Banner-University Medical Center, Tucson for further management. Upon arrival, the patient was taken immediately to the operating room for exploratory laparotomy.
Surgical observation: both gas and bile-stained fluid were observed in the abdomen. The bowel loops were dilated and the distal ileum showed wall thickening. After separation of the omentum and the loops of bowel, an inflammatory, perforated mass was observed on the mesenteric side of the ileum ~10 inches proximal to the ileocecal valve. A concern of perforated small bowel tumor versus diverticulum was raised. Appropriate segment proximal and distal to the mass were resected. The mesentery was divided with the LigaSure device. The resected specimen was submitted for pathology examination. Following surgery, the patient was recovered well and no further complications were reported.
Pathologic findings: the received specimen labeled as ‘perforated small bowel tumor’ was a segment of small bowel measuring 23.0 cm in length and 2.5–3.5 cm in diameter with an attached mesentery 3.5 cm in width throughout the entire length of the bowel. There was a tan-white, firm, irregular mesenteric lesion measuring 3.5 × 2.5 × 1.7 cm and extending into the bowel wall. The corresponding mucosal surface had a slightly elevated, well demarcated, round lesion 2.0 cm in diameter with central ulceration and a fistula going through the center of the mucosal lesion to the mesenteric lesion. There was no obvious diverticulum identified. The rest of the mucosa and bowel was unremarkable.
Microscopic examination of the mucosal lesion showed the presence of completely differentiated gastric mucosa with fundic type glands and surface foveolar epithelium (Fig. 1a). The fundic glands composed of specialized parietal cells and chief cells (Fig. 1b). In the center area of the GH, the mucosa was completely ulcerated with transmural inflammation and tissue necrosis forming a fistula tract surrounded by granulation tissue (Fig. 1c). At the edge of the ulceration, the gastric mucosa transited from fundic type into antral/pyloric type showing only mucinous cells in the deeper glands (Fig. 1d). The surrounding mesenteric fat had marked inflammation with fat necrosis forming mass-like lesion. However, there was no neoplasm or tumor identified. The final diagnosis was GH with ulceration and perforation.
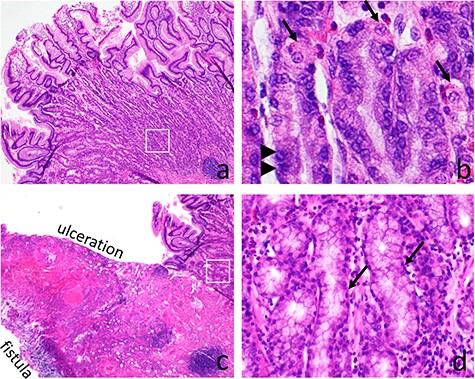
Gastric heterotopia (GH) causing bowel perforation. The GH shows full thickness of gastric fundic type mucosa with surface foveolar epithelium and deep fundic glandes (a). The fundic glands have specialized parietal cells (arrows) and chief cells (arrow heads) (b). At the center of the GH, mucosal ulceration with transmural inflammation and fistula formation are present (c). Adjacent to the ulcerative area, the mucosa transits into antral/pyloric type with only mucinous epithelium present in the deep glands (d)
DISCUSSION
Here, we report a case of ileal GH complicated with bowel perforation and mass formation clinically mimicking a neoplastic process in a 31-year-old man. Pathologic examinations provide the definitive diagnosis and show the presence of ectopic gastric mucosa with ulceration causing bowel perforation. This is an uncommon GH case for its unique presentation in distal ileum with bowel perforation and mass formation in an adult patient.
GH was first described in proximal esophagus in an autopsy by Schmidt in 1805 [3]. The most common site of GH is esophagus, especially the postcricoid portion of the esophagus at or just below the upper esophageal sphincter [2]. However, GH has been reported in almost every anatomic site along the GI tract from epiglottis to anus, and even in extremely unusual areas like the umbilicus, mediastinum or scrotum [4]. There are two proposed theories for the development of GH: one is congenital heteroplasia during organogenesis and another is acquired metaplasia during regeneration of injured epithelial [1]. In the concept of heteroplasia, GH in the foregut is considered as the congenital failure of developmental descent of the stomach. GH distal to the foregut is explained by the capability of the pluripotent stem cells of the endodermis to differentiate into gastric mucosa in the lower GI tract [5]. On the contrary, gastric metaplasia is an acquired adaptive change of the original anatomic cells into gastric mucosal cells in response to the local injury and/or inflammation [1]. Histologically, the difference between heteroplasia and metaplasia is the presence of distinct full thicknesses of gastric mucosa in the heteroplasia but usually only foveolar epithelium intermixed with the original site’s mucosa in metaplasia [2]. In this present case, the GH has a well demarcated full thickness of gastric mucosa suggesting its congenital heteroplasia nature. The stimulus for differentiation of gastric mucosa in the GI tract other than in stomach is unclear, likely associated with genetic regulation of defining genes such as Cdx2/Barx1 in the development of gastric phenotype [6].
GH in the GI tract is often an incidental finding endoscopically identified as a polyp, diverticula or ulcer [1]. Most symptomatic cases, especially congenital GH, are seen in children [1]. The presentations are usually mild such as abdominal pain and intestinal bleeding. A few GH cases are associated with intussusception, bowel obstruction, perforation and even adenocarcinoma [7, 8]. Surgical or endoscopic excision is generally the treatment of choice for GH and usually completely cures this disease. GH may be managed conservatively by histamine-2 receptor blockers [1, 2]. However, this present case demonstrates that GH has the potential to cause life-threatening complications such as bowel perforation and needs proper diagnosis and management.
FUNDING
This study is supported by the Department of Pathology at Banner-University Medical Center, College of Medicine, University of Arizona, Tucson, Arizona, USA.
CONFLICT OF INTEREST STATEMENT
None declared.
ETHICAL STATEMENT
This case study follows the regulations of the Internal Review Board for human research at the Banner-University Medical Center and the University of Arizona.



