-
PDF
- Split View
-
Views
-
Cite
Cite
Paul Burchard, Alan A Thomay, Resection of a colonic mass following trauma: a diagnostic dilemma, Journal of Surgical Case Reports, Volume 2020, Issue 7, July 2020, rjaa226, https://doi.org/10.1093/jscr/rjaa226
Close - Share Icon Share
Abstract
A 53-year-old Caucasian male presented with a 2-week history of abdominal distension, pain, nausea and lethargy. His symptoms began 1 day after an all-terrain vehicle accident during which he suffered blunt-force trauma to his mid-right abdomen. CT scan demonstrated abnormal thickening of the ascending colon and terminal ilium with surrounding inflammation within the retroperitoneum and colonic mesentery. Given his likely mechanism and symptomatic improvement, he was initially managed conservatively. However, he was readmitted with recurrence of symptoms, and a repeat CT scan demonstrated no interval improvement. An exploratory laparotomy was performed and a firm, fixed mass of the right-colon and colonic mesentery was found. Final histopathology of the mass revealed a diffuse lymphoid infiltrate with numerous mitotic figures and apoptotic cells. Immunohistochemical staining was positive for CD45, CD20, CD10, and BCL-6 and negative for CD3, TdT, and BCL-2, indicating a diagnosis of Burkitt lymphoma.
CASE PRESENTATION
A 53-year-old Caucasian male presented with a 2-week history of abdominal distension, pain, nausea and lethargy. His symptoms began 1 day after an all-terrain vehicle accident during which he suffered blunt-force trauma to his mid-right abdomen. Past medical history was significant for chronic left lower extremity deep vein thrombosis (DVT), managed with warfarin. Initial CT scan demonstrated abnormal thickening of the ascending colon and terminal ileum with surrounding inflammation within the retroperitoneum and colonic mesentery without perforation or abscess (Fig. 1). Further workup included a colonoscopy that revealed a portion of the ascending colon and cecum with edematous and erythematous mucosa. Biopsies showed unremarkable colonic mucosa with benign lymphoid aggregates. The patient was diagnosed with a likely hematoma of the right abdomen and was treated with nasogastric decompression and bowel rest. Given symptomatic improvement and belief that findings were secondary to trauma, conservative management was continued and the patient was discharged home in good condition. However, the patient was readmitted with recurrence of abdominal distention, pain and new-onset emesis. The patient continued to have bowel movements and pass flatus; however, physical exam was remarkable for worsening right-sided abdominal distention and rigidity. Repeat CT scan demonstrated no interval improvement in imaging findings and the decision was made for operative intervention due to increasing severity of symptoms, inability to tolerate oral intake and concerns for bowel obstruction.
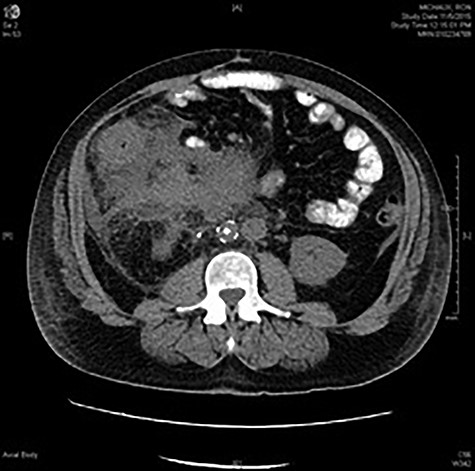
CT abdomen and pelvis with oral contrast at the level of L2 showing marked inflammatory changes surrounding the ascending colon and obscuring the colonic margins. Phlegmonous changes are seen extending to the root of the mesentery, in the left perinephric space and inferiorly into the pelvis. Infrarenal IVC filter and right ureteral stent are in place.
An exploratory laparotomy was performed and a firm, fixed, extra-luminal mass of the right-colon and colonic mesentery measuring 20 cm in diameter was found. A right hemicolectomy was performed along with excision of the mesentery, and gastrointestinal tract continuity was restored with a functional end-to-end anastomosis of the distal ileum to the transverse colon. The patient recovered slowly, but uneventfully without major complications.
In this patient, final histopathology of the mass arising from the right-colon and colonic mesentery revealed a diffuse lymphoid infiltrate with numerous mitotic figures and apoptotic cells creating a classic ‘starry sky’ appearance (Fig. 2). Immunohistochemical staining was positive for CD45, CD20, CD10, and BCL-6 and negative for CD3, TdT, and BCL-2, indicating a diagnosis of Burkitt lymphoma (BL).
DISCUSSION
This pattern is classic for BL, a highly aggressive non-Hodgkin lymphoma that can present in the form of three major clinical variants: endemic, sporadic and HIV-associated (Table 1) [1]. Diagnosis of BL is performed by pathologic evaluation of tumor samples. The histological appearance of BL has been described a classical ‘starry sky’ appearance that occurs due to a simultaneously high rate of proliferation and apoptosis and subsequent engulfment of apoptotic cells by histiocytic cells present within the tumor [2]. BL is characterized by dysregulation of the c-MYC oncogene, which not only drives enhanced cellular division and metabolism, but also results in uncoordinated apoptosis [2]. BL accounts for only 1% of adult non-Hodgkin lymphomas, and presents as a bulky abdominal mass in up to 90% of cases involving lymphoid tissues of the gut (including the cecum, terminal ileum and colon) and also may involve the kidneys and other abdominal viscera [1, 3]. Up to 70% of cases feature bone marrow involvement and 40% present with tumor penetration into the central nervous system (CNS) [2]. Patients also commonly present with systemic B-symptoms and evidence of tumor lysis including elevated LDH and uric acid levels.
| Burkitt lymphoma variants . | Population affected . | Epidemiology . | Characteristics . |
|---|---|---|---|
| Endemic | Equatorial Africa & New Guinea |
|
|
| |||
| |||
| Sporadic | USA and Western Europe |
|
|
|
| ||
|
| ||
| |||
| |||
| HIV-associated | HIV patients |
|
|
|
|
| Burkitt lymphoma variants . | Population affected . | Epidemiology . | Characteristics . |
|---|---|---|---|
| Endemic | Equatorial Africa & New Guinea |
|
|
| |||
| |||
| Sporadic | USA and Western Europe |
|
|
|
| ||
|
| ||
| |||
| |||
| HIV-associated | HIV patients |
|
|
|
|
| Burkitt lymphoma variants . | Population affected . | Epidemiology . | Characteristics . |
|---|---|---|---|
| Endemic | Equatorial Africa & New Guinea |
|
|
| |||
| |||
| Sporadic | USA and Western Europe |
|
|
|
| ||
|
| ||
| |||
| |||
| HIV-associated | HIV patients |
|
|
|
|
| Burkitt lymphoma variants . | Population affected . | Epidemiology . | Characteristics . |
|---|---|---|---|
| Endemic | Equatorial Africa & New Guinea |
|
|
| |||
| |||
| Sporadic | USA and Western Europe |
|
|
|
| ||
|
| ||
| |||
| |||
| HIV-associated | HIV patients |
|
|
|
|
Despite the highly aggressive nature of BL, these tumors tend to be exceptionally chemosensitive and potentially curable with appropriate combination chemotherapy [2]. As BL can involve the CNS in a significant proportion of cases, systemic treatment is combined with instillation of intrathecal chemotherapy for CNS prophylaxis [2, 4]. As a result of BL’s high aggression and sensitivity to chemotherapy, patients can develop tumor lysis syndrome after the initiation of treatment. Tumor lysis syndrome is an oncologic emergency due to massive tumor cell lysis, resulting in the release of large amounts of intracellular potassium, phosphate, and uric acid into the systemic circulation. Prevention and management of this complication requires intravenous fluids, hyperuricemic agents (allopurinol, rasburicase, febuxostat) and close monitoring of serum electrolytes, phosphorous, and uric acid [5].
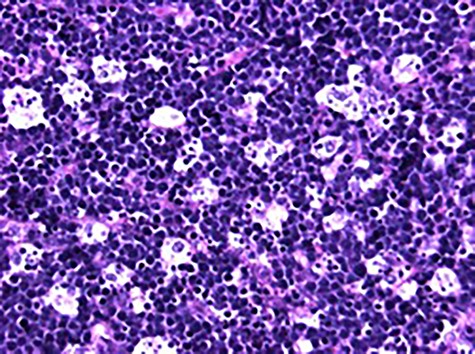
High power photomicrograph (40×) of H&E stained tumor sample showing diffuse lymphoid infiltrate with numerous mitotic figures and apoptotic cells creating the classic ‘starry sky’ appearance of BL.
Surgical removal of the tumor is not typically necessary and radiotherapy has not been shown to improve survival. However, surgical management of BL may be indicated to establish diagnosis, or in instances of obstruction, perforation, fistula formation or uncontrollable hemorrhage [4–6]. Some studies in the pediatric population have argued for localized resection in patients with intra-abdominal BL who present with limited disease [7–9]. Complete resection may avoid potential complications, allows for less intensive chemotherapy, and can improve quality of life [7–9]. In cases of diffuse disease, surgical interventions should be limited to biopsy as aggressive resection is often unnecessary and unsafe [7–9]. A summary of the characteristics, diagnosis and management of BL can be found in Table 2 [10].
| Symptoms . | Clinical features . | Diagnosis . | Pathology . | Treatment . |
|---|---|---|---|---|
|
|
|
|
|
|
|
| ||
|
| |||
|
|
| Symptoms . | Clinical features . | Diagnosis . | Pathology . | Treatment . |
|---|---|---|---|---|
|
|
|
|
|
|
|
| ||
|
| |||
|
|
| Symptoms . | Clinical features . | Diagnosis . | Pathology . | Treatment . |
|---|---|---|---|---|
|
|
|
|
|
|
|
| ||
|
| |||
|
|
| Symptoms . | Clinical features . | Diagnosis . | Pathology . | Treatment . |
|---|---|---|---|---|
|
|
|
|
|
|
|
| ||
|
| |||
|
|
This patient was diagnosed with stage IV colonic and mesenteric BL and was subsequently treated with standard of care chemotherapy regimen, which consisted of R-HyperCVAD (rituximab, cyclophosphamide, vincristine, doxorubicin and dexamethasone) alternating with R-HD MTX/Ara-C (rituximab, high dose methotrexate and cytarabine) with appropriate intrathecal therapy. The patient’s baseline and post-treatment laboratory values are as demonstrated in Table 3. His post-treatment PET/CT was consistent with a complete response and he continues to follow with our medical oncology colleagues every 6 months. Now 4 years removed from therapy, he remains asymptomatic without evidence of relapse.
| Laboratory value . | Baseline . | Post treatment . |
|---|---|---|
| CBC and differential | ||
| WBC | 7.8 | 5.9 |
| Hemoglobin | 10.1 (low) | 14.2 |
| Hematocrit | 30.4 (low) | 41.0 |
| Platelet count | 183 | 136 (low) |
| Lymphocyte count | 0.878 (low) | 1.27 |
| BMP | ||
| Sodium | 142 | 140 |
| Potassium | 4.0 | 4.2 |
| Chloride | 106 | 104 |
| Carbon dioxide | 19 (low) | 30 |
| Anion gap | 17 (high) | 4 |
| Creatinine | 2.60 (high) | 0.99 |
| BUN | 38 (high) | 14 |
| LDH | 292 (high) | 192 |
| Uric acid | 3.9 | 5.8 |
| Laboratory value . | Baseline . | Post treatment . |
|---|---|---|
| CBC and differential | ||
| WBC | 7.8 | 5.9 |
| Hemoglobin | 10.1 (low) | 14.2 |
| Hematocrit | 30.4 (low) | 41.0 |
| Platelet count | 183 | 136 (low) |
| Lymphocyte count | 0.878 (low) | 1.27 |
| BMP | ||
| Sodium | 142 | 140 |
| Potassium | 4.0 | 4.2 |
| Chloride | 106 | 104 |
| Carbon dioxide | 19 (low) | 30 |
| Anion gap | 17 (high) | 4 |
| Creatinine | 2.60 (high) | 0.99 |
| BUN | 38 (high) | 14 |
| LDH | 292 (high) | 192 |
| Uric acid | 3.9 | 5.8 |
| Laboratory value . | Baseline . | Post treatment . |
|---|---|---|
| CBC and differential | ||
| WBC | 7.8 | 5.9 |
| Hemoglobin | 10.1 (low) | 14.2 |
| Hematocrit | 30.4 (low) | 41.0 |
| Platelet count | 183 | 136 (low) |
| Lymphocyte count | 0.878 (low) | 1.27 |
| BMP | ||
| Sodium | 142 | 140 |
| Potassium | 4.0 | 4.2 |
| Chloride | 106 | 104 |
| Carbon dioxide | 19 (low) | 30 |
| Anion gap | 17 (high) | 4 |
| Creatinine | 2.60 (high) | 0.99 |
| BUN | 38 (high) | 14 |
| LDH | 292 (high) | 192 |
| Uric acid | 3.9 | 5.8 |
| Laboratory value . | Baseline . | Post treatment . |
|---|---|---|
| CBC and differential | ||
| WBC | 7.8 | 5.9 |
| Hemoglobin | 10.1 (low) | 14.2 |
| Hematocrit | 30.4 (low) | 41.0 |
| Platelet count | 183 | 136 (low) |
| Lymphocyte count | 0.878 (low) | 1.27 |
| BMP | ||
| Sodium | 142 | 140 |
| Potassium | 4.0 | 4.2 |
| Chloride | 106 | 104 |
| Carbon dioxide | 19 (low) | 30 |
| Anion gap | 17 (high) | 4 |
| Creatinine | 2.60 (high) | 0.99 |
| BUN | 38 (high) | 14 |
| LDH | 292 (high) | 192 |
| Uric acid | 3.9 | 5.8 |
CONCLUSION
BL can present in the form of three major clinical variants: endemic, sporadic and HIV-associated. CT scan and colonoscopy findings can be largely non-specific and difficult to interpret. Therefore, clinical suspicion must remain high in patients presenting with abdominal pain and systemic complaints. In some patients, surgical intervention is required for diagnosis or emergent treatment. Once identified, BL is highly responsive to appropriate combination chemotherapy.
FUNDING
No funding was required.
CONFLICT OF INTEREST STATEMENT
None declared.
DISCLOSURE STATEMENT
The authors have nothing to disclose.
References
- computed tomography
- immunohistochemistry
- inflammation
- burkitt's lymphoma
- accidents
- cd20 antigens
- cd45 antigens
- colonic neoplasms
- dna nucleotidylexotransferase
- bcl2 gene
- mesentery
- nausea
- neprilysin
- pain
- retroperitoneal space
- european continental ancestry group
- wounds and injuries
- abdomen
- colon
- diagnosis
- lethargy
- abdominal swelling
- laparotomy, exploratory
- ascending colon
- all-terrain vehicles
- infiltrates
- histopathology tests



