-
PDF
- Split View
-
Views
-
Cite
Cite
Jorn P Meekel, Usha K Coblijn, Marcel J Flens, Sandra Muller, Frank C Boer den, Small bowel obstruction caused by 18FDG-negative ileocecal metastasis of lobular breast carcinoma, Journal of Surgical Case Reports, Volume 2020, Issue 7, July 2020, rjaa167, https://doi.org/10.1093/jscr/rjaa167
Close - Share Icon Share
Abstract
Breast carcinoma is the most frequently diagnosed cancer in women. In up to 30%, distant metastases will occur; however, ileocecal metastases are rare. Although there have been cases reported that demonstrate ileocecal metastases of breast carcinoma, PET/CT-negative cases have never been described. We present a patient with a small bowel obstruction, preoperatively complicated by pulmonary embolisms. The patient underwent placement of an inferior vena cava filter followed by hemicolectomy. Pathological examination revealed ileocecal lobular breast carcinoma metastases and adjacent peritoneal carcinomatosis, which had shown no intestinal 18FDG uptake 7 weeks prior to presentation. Subsequently, symptoms of metastases and the paraneoplastic syndrome progressed, and the patient was referred to the medical oncologist for palliative therapy. Although uncommon, physicians should be aware of potential presence of 18FDG-negative gastrointestinal metastases of breast cancer.
INTRODUCTION
Breast cancer is the most common malignancy diagnosed in women worldwide (11.6%) [1]. Common metastatic locations of infiltrative breast cancer are the skeletal system, the liver, the lung, the brain and the ovaries [2]. Metastasis to the intestinal tract is uncommon. McLemore et al. showed that in only 0.6% of patients with metastases, these were pathologically confirmed in the gastrointestinal tract or peritoneum, mostly following primary lobular carcinoma [3].
Positron emission tomography (PET)/CT has a high sensitivity and specificity in the diagnosis of metastatic breast cancer [4]. Although a paraneoplastic syndrome is regularly observed in oncological patients, the risk of pulmonary embolism in comorbidity with breast cancer is relatively low (1.5%) [5]. Nonetheless, the risk of venous thromboembolism (VTE) increases approximately 58-fold in patients with metastatic cancer compared to patients without cancer [6]. We report the case of a female patient who presented herself with a clinical intestinal obstruction and presumably paraneoplastic symptoms caused by metastasis from infiltrative lobular breast carcinoma.
CASE REPORT
A 64-year-old female was diagnosed with a left-sided cT2N1M0 lobular breast carcinoma for which she was treated with neoadjuvant chemotherapy. Partial radiological response was observed (Fig. 1) and an oncoplastic breast-conserving surgery was performed. After pathological examination, an ypT2N1a(sn) TNM classification (8th edition UICC), ER-positive (100%), PR-negative (0%) and HER2-negative, was found, and adjuvant radiotherapy and hormone therapy were indicated.
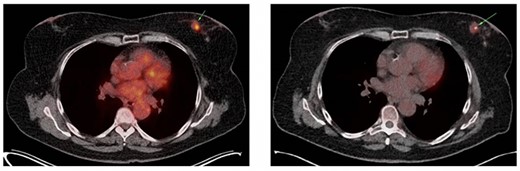
(A) Axial slice of a thoracic PET/CT showing the lobular carcinoma in the left mamma (green arrow) before neoadjuvant chemotherapy. This PET/CT was performed 7 months prior to ER presentation. (B) Axial slice of a thoracic PET/CT showing the lobular carcinoma in the left mamma (green arrow) after neoadjuvant chemotherapy with partial response. This PET/CT was performed 7 weeks prior to ER presentation.
The patient was admitted to the emergency department 23 days after the oncoplastic breast surgery. She presented with abdominal pain, nausea, emesis and stool changes. General examination was normal and vital signs were normal except tachycardia. Abdominal distension, high-pitched tinkling bowel sounds and epigastric tenderness were observed. Laboratory tests revealed a white blood cell (WBC) count of 8.8*10E9/L and C-reactive protein (CRP) level of 19.4 mg/L. Abdominal computed tomography (CT) scan showed a short bowel obstruction caused by a stenosis in the terminal ileum and ileocecal junction without lymphadenopathy signs or metastasis. The basal lung segments were suspect for bilateral pulmonary embolisms (Fig. 2A–C), which was confirmed by a thoracic CT angiography. Retrospectively, an ileocecal tumor could not be identified on a follow-up PET/CT scan which was performed 7 weeks prior to emergency room presentation (Fig. 2D).
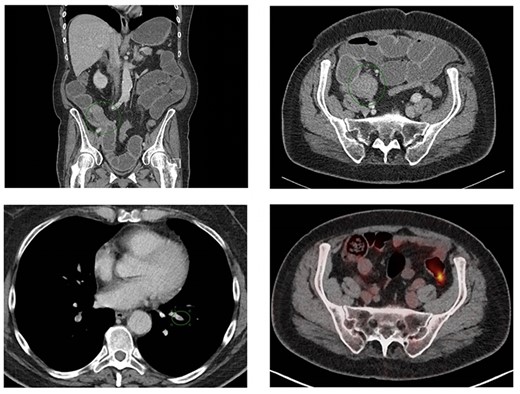
Coronal (A) and axial slice (B) of the abdominal CT scan at ER presentation showing small bowel obstruction with terminal ileum (green ellipse) and ileocecal junction stenosis. (C) CT scan at ER presentation also showed pulmonary embolisms in basal lung segments bilaterally (green ellipse). (2D) PET/CT performed 7 weeks prior to ER presentation showed no 18FDG uptake terminal ileum or ileocecal junction.
The following day, she underwent inferior vena cava filter (VCF) placement after which a right-sided hemicolectomy and creation of an end ileostomy were performed. During surgery, there was an abnormal tissue spot on the peritoneum, which was suspect for a metastasis. After complete abdominal cavity evaluation, the peritoneal carcinomatosis index was judged to be 2. Pathological examinations revealed stenoses in the terminal ileum and the cecum, caused by multiple loci of metastases of lobular breast carcinoma (Fig. 3) and peritoneal carcinomatosis. Resection margins were free from tumor cells, and there was no metastasis in 15 lymph nodes and omentum.
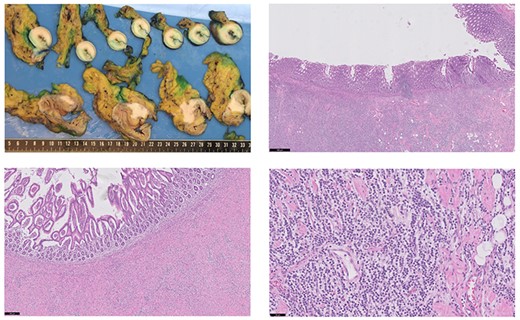
Macroscopic findings (A): in the longitudinal section of the cecum, there was no mucosal ulceration found. In the terminal ileum a stenosis was observed due to a submucosal circular pale mass. In the colonic wall and mesocolon, a hard pale mass was found too. Microscopic examination of the terminal ileum (B) and colon (C) showed that the mucosal layer was intact. The tumor cells with linear growth and loss of cohesion (D) were spread from the serosal to the submucosal layer (hematoxylin and eosin staining). Black bar indicates 500, 200 and 50 μm for Fig. 3B–D, respectively. The unexpected histological finding with lobular features, similar to the oncoplastic breast-conserving surgery specimen of the left breast, prompted further immunohistochemical study. In both the terminal ileum and in the cecum, the tumor cells were strongly positive for cytokeratin 7 (CK7), GATA-3 and estrogen receptor (ER). The tumor cells were negative for HER2 and progesterone receptor (PR) (similar to the pattern found in in the breast tumor). The markers of enteric differentiation: cytokeratin 20 (CK20), CDX-2 and carcinoembryonic antigen (CEA) were all negative. The positive staining of GATA-3, ER and CK7 is highly consistent with metastatic disease from the primary breast carcinoma.
Despite continuous use of anticlotting agents, it was impossible to remove the inferior VCF twice (5 and 22 days postoperatively) due to thrombosis adjacent to the VCF. Eight days postoperatively, the patient had progressive pain in the left lower abdominal quadrant, stoma bleeding, tachycardia, hypotension and a hemoglobin level of 5.8 g/dL (3.6 mmol/L), WBC count of 36.2*10E9/L and CRP level of 32.9 mg/L. CT angiography revealed a rectus sheath hematoma with continuation into the small pelvic cavity without active blush, while heparin was administrated (Fig. 4A). Intravenous heparin was switched to therapeutic subcutaneous nadroparin and because of an infected hematoma suspicion, piperacillin/tazobactam was given. Ca15-3 was 49 and 38 kU/L (reference range 0–30 kU/L) at the 21st and 24th postoperative day, respectively.
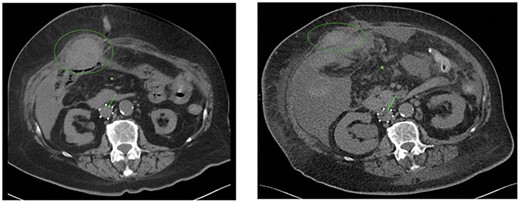
CT scans performed at the 8th (A) and 25th (B) postoperative day showed a decreasing rectus sheath hematoma (green ellipse), increasing free intraperitoneal fluid (green asterisk) and blood clot progression with small air configuration near the VCF (green arrow). Also, enlargement of the blood clot at the level of the VCF, an increase in pleural fluid, bile duct dilatation and an increase in right-sided hydronephrosis were observed caused by further metastatic disease and paraneoplastic syndrome.
Due to therapy-resistant pulmonary embolisms, recurrent glucose level swings, progressive renal insufficiency and the indication for palliative hormonal therapy, the patient was transferred to the internal medicine ward for further treatment. After clinical deterioration, an abdominal CT scan 25 days postoperatively showed a gradual decrease of the rectus sheath hematoma with concomitant increase in free intraperitoneal fluid presumably following perforation of the hematoma and new abnormal findings as a consequence of further metastases and the paraneoplastic syndrome (Fig. 4B). Therefore, maximum conservative palliative treatment was instituted, patient died shortly thereafter, and no autopsy was performed.
DISCUSSION
Globally, breast cancer is the most frequently diagnosed malignancy in women, and it is the leading cause of oncologic deaths in women [1]. Lobular carcinomas account for about 15% of all breast cancers, and the remainder of the cases are mainly ductal carcinomas [7]. Gastrointestinal metastasis of infiltrating breast carcinoma is rare. McLemore et al. studied 12 001 patients with metastatic breast cancer and found 24, 15, 10 and 4 cases for metastases in colon/rectum, stomach, small intestine and esophagus, respectively. Infiltrating lobular carcinoma represented 64% of all gastrointestinal metastases, infiltrating ductal carcinoma 32% and a mix of both types 2% [3].
Consistent with previous case reports, we found that symptomatic ileocecal metastases present with small bowel obstruction [8]. In another case report, suspicion of ileal metastasis was raised during a colonoscopy for hematochezia and, in retrospect, fluorodeoxyglucose F 18 (18FDG) uptake in the terminal ileum was observed, but these findings were attributed to inflammation [9].
In literature, it has been shown that sensitivity and specificity for PET/CT in the diagnosis of primary breast cancer are low. Nonetheless, in this case the primary tumor showed 18FDG uptake. However, the abdominal metastases were not detected on PET/CT, despite high overall sensitivity (0.96) and specificity (0.95) in metastatic breast cancer disease [4]. Although still high, sensitivity and specificity are lower in lobular carcinoma than in ductal carcinoma.
In women with breast cancer, there is a 3- to 4-fold increased risk of VTE for which the main risk factors are chemotherapy and presence of metastatic disease (paraneoplastic). This risk remains elevated after chemotherapy cessation, and there is a particularly increased risk perioperatively. Therefore, under these circumstances, prophylaxis for higher-risk outpatient cancer patients should be considered [10].
Although the occurrence of gastrointestinal metastasis in breast cancer is low, one should be aware of this possibility. In patients with a history of breast cancer presenting with anemia, stool changes, bowel obstruction, melena or hematochezia, imaging of the gastrointestinal tract is mandatory.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.



