-
PDF
- Split View
-
Views
-
Cite
Cite
Takashi Miyata, Hiroyuki Takamura, Ryosuke Kin, Hisashi Nishiki, Akifumi Hashimoto, Yoritaka Fujii, Seiko Miura, Jun Fujita, Daisuke Kaida, Yasuto Tomita, Naohiko Nakamura, Hideto Fujita, Shinichi Kinami, Nobuhiko Ueda, Takeo Kosaka, Pancreatic neuroendocrine tumor featuring growth into the main pancreatic duct and tumor thrombus within the splenic vein: a case report, Journal of Surgical Case Reports, Volume 2020, Issue 7, July 2020, rjaa155, https://doi.org/10.1093/jscr/rjaa155
Close - Share Icon Share
Abstract
A 48-year-old woman was admitted to our hospital because of upper abdominal pain. Computer tomography showed an enhancing mass in the pancreatic body, dilation of the main pancreatic duct (MPD) and a filling defect within the splenic vein. On the basis of the preoperative diagnosis of pancreatic body cancer, distal pancreatectomy was scheduled. The pancreas was divided along the left edge of the gastroduodenal artery; however, frozen pathological examination of the pancreatic stump was tumor positive, and therefore a total pancreatectomy was performed. The lesion was a white expansive nodular mass that had spread into the MPD and protruded into the splenic vein. A pathological diagnosis of non-functioning neuroendocrine tumor (NET) was made. In general, imaging findings of disruption of the MPD and tumor vein thrombus are characteristics of pancreatic ductal adenocarcinoma, but are uncommon in NET. However, NET should be included in the differential diagnosis for such patients.
INTRODUCTION
Pancreatic neuroendocrine tumors (PNETs) are relativity rare pancreatic tumors, accounting for only 1–3% of all pancreatic neoplasms, and can be divided into functioning and non-functioning tumors. Non-functional PNETs account for approximately 15% of PNETs [1]. Unlike functioning PNETs, due to the lack of endocrine symptoms, preoperative diagnosis of patients with non-functional PNETs is usually imaging based. However, imaging findings of growth into the main pancreatic duct (MPD) and simultaneous tumor thrombus in the splenic vein are extremely rare features of a non-functional PNET. Herein, we report a case of PNET that was difficult to diagnose preoperatively.
CASE REPORT
A 48-year-old woman was referred to our hospital because of upper abdominal pain. Her medical and family histories were unremarkable, and her abdomen was soft, with no palpable mass. Laboratory investigation revealed slight leukocytosis (white blood cell count 10 280/μl), but other data, including the serum concentrations of tumor markers, immunoglobulin (Ig) G and IgG4, and pancreatic hormones (gastrin, insulin, glucagon, somatostatin, and pancreatic peptide) were all within their normal ranges.
Abdominal-enhanced computed tomography (CT) revealed a low-density pancreatic body tumor of 25 mm in diameter, which was close to the gastroduodenal artery (GDA), and was accompanied by atrophy of the distal pancreatic parenchyma and dilation of the upstream MPD (Fig. 1A). Moreover, CT imaging of the portal phase revealed a filling defect within the splenic vein where it was in contact with the tumor (Fig. 1B). On magnetic resonance imaging, the tumor appeared as an area of signal hyperintensity on T2-weighted and diffusion-weighted images (Fig. 2A and B). On positron emission tomography-CT scan, the tumor exhibited greater uptake of 18F-fluorodeoxyglucose (Fig. 3). Although a definitive preoperative diagnosis could not be made, we suspected pancreatic ductal adenocarcinoma, and distal pancreatectomy with lymph node dissection was scheduled, without neoadjuvant chemotherapy, after obtaining consent from the patient. The pancreas was divided along the left edge of the GDA after mobilization of the artery; however, pathological examination of a frozen section of the pancreas stump was positive for PNET, and therefore a total pancreatectomy was performed (Fig. 4). The duration of surgery was 610 min, the intraoperative blood loss was 195 mL, and blood transfusion was not performed.
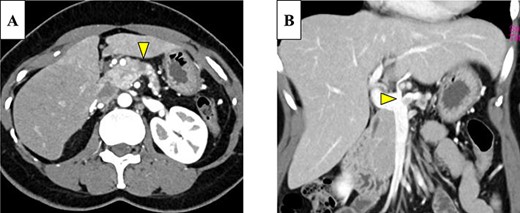
(A) Enhanced computed tomography (CT) showed a 25-mm nonhypervascular enhancing mass in the pancreatic body, which was accompanied by atrophy of the distal pancreatic parenchyma, and the upstream main pancreatic duct (MPD) was dilated to 11 mm in diameter (yellow arrow). (B) The mass also invaded the splenic vein (yellow arrow).
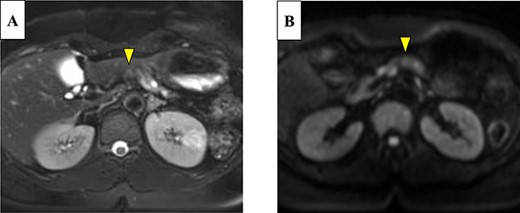
On magnetic resonance imaging, the pancreatic tumor is shown as a hyperintense area in T2- (A) and (B) diffusion-weighted images (yellow arrows).
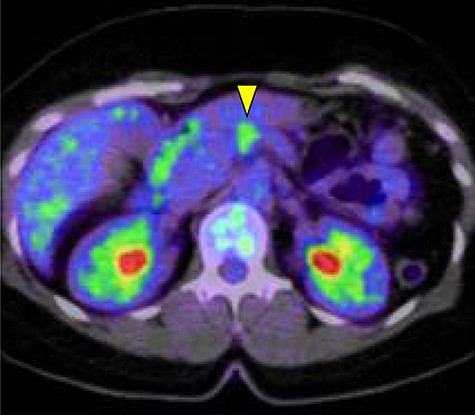
The pancreatic tumor exhibited greater uptake of 18F-fluorodeoxyglucose (standardized uptake value of 3.8) on positron emission tomography (yellow arrow).
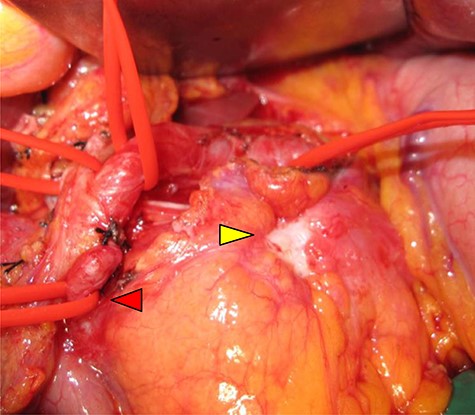
Intraoperative view: serosal exposure of the tumor was confirmed in the pancreatic body (yellow arrow), and the gastroduodenal artery was shown (red arrow).
The gross appearance of the resected specimen was of a white, solid, nodular tumor measuring 25 × 15 mm, which was located in the pancreatic body, with spread into the MPD and protrusion into the splenic vein (Fig. 5A and B). Microscopically, the tumor largely comprised fibrovascular stroma, with small nests and cords of uniform cells arranged in a rosette-like pattern (Fig. 6A and B). Immunohistochemistry showed that the Ki-67 labeling index was 5%, and there was positive staining for chromogranin A and synaptophysin. Therefore, a diagnosis of non-functioning PNET G2 was made and one of the 32 lymph nodes examined was tumor positive. The patient was discharged 30 days after surgery, having experienced no complications, and no tumor recurrence occurred in the 13 months following, in the absence of adjuvant therapy.
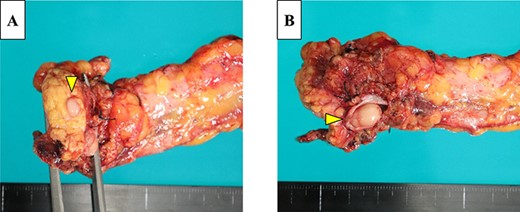
The resected pancreas contained a yellow mass that extended within the lumen of the MPD (A) and tumor thrombus in the splenic vein (B).
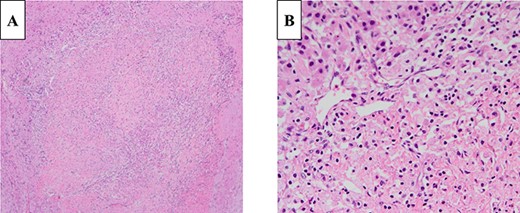
Histologically, the tumor was composed of cells containing ellipsoidal eccentric nuclei and eosinophilic cytoplasm in a rosette-like arrangement (A: 100×, B: 400×; H&E staining).
DISCUSSION
Non-functional PNET is a potentially highly malignant but relatively rare tumor, and due to the lack of endocrine symptoms associated, a diagnosis may only be made when the tumor is large and causes symptoms such as abdominal pain or obstructive jaundice [2]. Preoperative diagnosis is usually made using image modalities. In the present report, we present an extremely rare case of a patient with a non-functioning NET of the pancreatic body that was difficult to diagnose because of two atypical imaging findings and was treated by surgical resection.
PNETs are normally hyper-vascular tumors that are typically well-defined, often grow expansively, and cause displacement and deviation of the MPD on contrast-enhanced CT [3]. However, this type of tumor rarely involves the pancreatic ductal system, and therefore, growth into the MPD is a rare feature [4], as is tumor thrombus [5, 6]. To the best of our knowledge, there has been only one report, by Kawakami et al. [7], published in the English literature of a patient with simultaneous intraductal growth and tumor thrombus caused by a PNET, as identified in the present case.
The present patient had imaging findings that differed from those of typical PNET, which is a non-hypervascular tumor, with intraductal growth having led to atrophy of the distal pancreatic parenchyma, dilation of the upstream MPD and splenic vein involvement. Although several previous reports have stated that endoscopic ultrasonography (EUS) or endoscopic retrograde cholangiopancreatography is helpful for the differential diagnosis of PNET [8], the two CT findings reported herein are more characteristic of pancreatic ductal adenocarcinoma, which made preoperative differentiation very difficult in the present case. Kawakami et al. [7] were also unable to differentiate pancreatic ductal adenocarcinoma and PNET preoperatively.
In general, PNETs are slow-growing and prolonged survival is likely if radical resection is performed immediately following diagnosis, before the appearance of distant metastases [1]. In contrast, pancreatic ductal adenocarcinoma is associated with a poor prognosis, and recently, several reports have described a significant survival benefit of the use of neoadjuvant chemotherapy in preference to surgery, even for patients with potentially resectable pancreatic ductal adenocarcinoma [9]. Therefore, accurate preoperative diagnosis is essential for the selection of the optimal treatment method. Therefore, EUS-guided fine needle aspiration should be considered for patients in whom it is difficult to make a preoperative definitive diagnosis [10].
Simultaneous findings of intraductal growth and tumor thrombus in patients with non-functional PNET are rare, and therefore, it is difficult to make a definitive diagnosis prior to surgery. However, PNET should be included in the differential diagnosis of patients with these two findings, and early treatment may be essential for them to have a good prognosis. We believe that the present case report will provide a useful guide for the clinical diagnosis and treatment of this disease.
CONSENT
The patient discussed in this case report provided their informed consent for the publication of their information.
ACKNOWLEDGEMENTS
We thank Mark Cleasby, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
FUNDING
No authors have direct or indirect commercial and financial interests associated with the publication of this article.



