-
PDF
- Split View
-
Views
-
Cite
Cite
Jay Lodhia, Alex Mremi, Jeremia J Pyuza, Nicholas Bartholomeo, Ayesiga M Herman, Schistosomiasis and cancer: Experience from a zonal hospital in Tanzania and opportunities for prevention, Journal of Surgical Case Reports, Volume 2020, Issue 5, May 2020, rjaa144, https://doi.org/10.1093/jscr/rjaa144
Close - Share Icon Share
Abstract
Schistosomiasis infection is endemic in many parts of Tanzania. The morbidity due to Schistosomiasis and its association with cancer remains to be of great concern and poses greater challenge that needs to be assessed. Cancer is an increasing public health problem in most sub-Saharan African countries, and yet, cancer control programs and the provision of early detection and treatment services are limited despite this increasing burden. This article aims to discuss case series of patients diagnosed with urinary bladder, prostate and colorectal cancer together with Schistosoma infection. We further highlight the opportunities for combating new Schistosomiasis infection, a potential to reduce its oncological complications particularly in low-resource setting.
INTRODUCTION
About one-third of all cancers in developing countries are associated with infectious diseases [1]. The observed morbidity and mortality due to cancer in the developing world have largely been attributed to exposure to parasitic, viral and bacterial infections [1]. Schistosomiasis is a neglected tropical disease (NTD), and in the 2010 Global Burden of Disease Study, NTDs accounted for 26.06 million disability-adjusted life years and at least 230 million people are estimated to have Schistosomiasis [2].
Schistosomiasis is endemic in most part of the country [3] with estimated prevalence of 51.5%; about 19 million people were affected in the year 2009 [3]. The past review from Tanzania highlighted severe areas, including around the Lake Zone, Dar es Salaam and Zanzibar, to be mostly affected by the disease [3]. Prevention schemes are laid down in most of the endemic areas, and it is a question whether these are practiced effectively [3].
Cancer burden is increasing in Tanzania [4]. The aim of this case series is to add to the current body of knowledge by providing our experience on three patients who were diagnosed with bladder, prostate and colorectal cancer associated with Schistosomiasis.
CASE REPORTS
Case number 1
A 78-year-old male a history of portal hypertension secondary to periportal fibrosis, on spironolactone and propranolol, presented with gradual onset of abdominal distension for 5 months. Distension was associated with pain and bilious non-blood-stained vomiting, failure to pass stool and flatus for 3 days.
He had a history of working in the rice fields for more than 30 years. No history of smoking or alcohol consumption.
On examination, patient was ill-looking, with Glasgow Coma Scale (GCS) of 10/15, dehydrated, pale, jaundiced, dyspneic and no peripheral lymphadenopathy. Vitals Blood Pressure (BP), 130/60 mm Hg, Pulse Rate (PR) 105 B/M, Respiratory Rate (RR) 30 B/M, temperature was 37.2 °C and oxygen saturation 92% RA. Per abdomen; distended with visible peristalsis soft, tender at the right hypochondriac region. Patient had positive shifting dullness and exaggerated bowel sounds on auscultation. On digital rectal examination, cardiopulmonary systems were unremarkable.
Upon investigation, Hemoglobin level (Hb) was 9.8 (13–17 g/dl), Na+ 138.8 (135–145 mmol/l), K+ 3.47 (3.5–5 mmol/l), serum urea 20.55 (1.2–3 mmol/l), serum creatinine 1.96 (0.8–1.3 mg/dl), serum total protein 32.03 g/l (60–80 g/l) and transaminase enzymes were within normal range. The working diagnosis was large bowel obstruction.
Radiological investigations
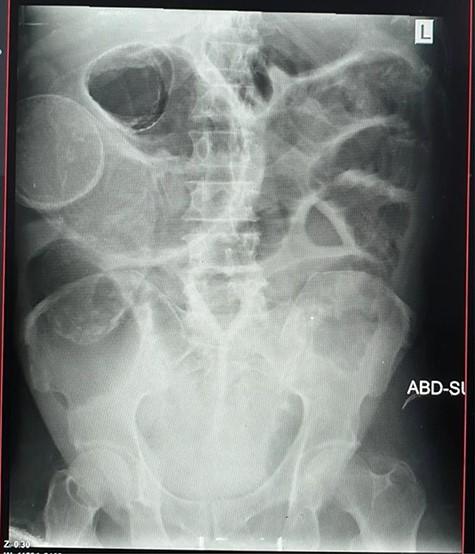
Plain abdominal X-ray (supine) showed grossly dilated large bowels (Fig. 1), and a chest X-ray showed a right-sided pneumothorax, which was managed by a thoracostomy tube (Fig. 2).
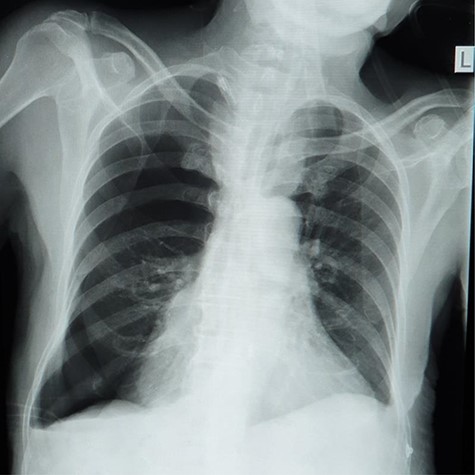
Chest X-ray showing right-sided pneumothorax with thoracostomy tube.
Abdominal ultrasound showed gaseous abdomen with dilated bowel loops, liver with course texture, heterogeneous margins, signs of portal fibrosis, minimal ascites and slightly splenomegaly.
Intraoperatively, 1.5 l ascitic fluid, distended ascending colon and a tumor of 2 × 4 cm at the hepatic flexure completely obstructing the lumen were noted. Multiple nodules were seen on the liver surface.
Resection of the lesion was done followed by double barrel colostomy sample was submitted for histopathological report.
The report revealed a segment of colon with low-grade invasive adenocarcinoma (pT3NxM1) and Schistosoma eggs embedded in the tumor (Fig. 3).
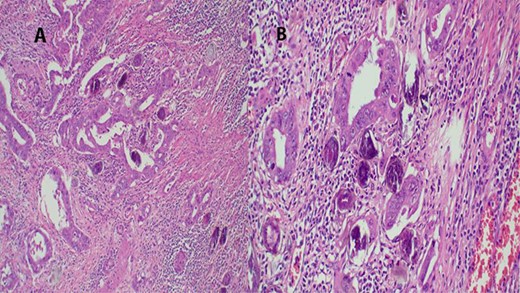
(A) Histopathology of colon with adenocarcinoma associated with Schistosoma ova with poorly formed granulomatous inflammation, hematoxylin and eosin (H&E) ×20 magnification. (B) Adenocarcinoma and Schistosoma ova with dense lymphocytic infiltrates ×40 magnification.
Patient was managed in intensive care unit; later, he developed sepsis and succumbed on the eighth post-operative day.
Case number 2
A 66-year-old man, a peasant, presented with inability to pass urine, history of weak stream, post-voiding dribbling and increase in frequency of urination more at night. He has lower abdominal distension with severe lower back pain for about 6 months. He denied any history of trauma, cigarette smoking or alcohol consumption. Patient recalls the history of blood in urine a year earlier, which resolved spontaneously.
On general examination, patient was alert, cachexic, severely pale, palpable submental and axillary lymph node and pitting lower limb edema. vital signs, Temp 35.6 (36.6–37°C), BP 112/70 mmHg, PR 85 B/M, RR 24 B/M.
Per abdomen, no distension or tenderness both superficial and deep palpation. The liver was enlarged 16 cm below costal margin. Normal bowel sounds were heard. On digital rectal examination, there was a hard asymmetrical prostate, multinodulated with obturated median sulcus.
Laboratory findings revealed the Hb level of 5.5 (13–17 g/dl), creatinine of 7.29 (0.8–1.3 mg/dl), serum urea of 26.29 (1.2–3 mmol/l), PSA of 774.5 (<4 ng/ml), urine culture showed mixed growth. K+ 5.13 (3.5–5 mmol/l) no ECG changes, Na+ 104.25 (135–145 mmol/l) and Cl+ 78.97 (95–105 mmol/l).
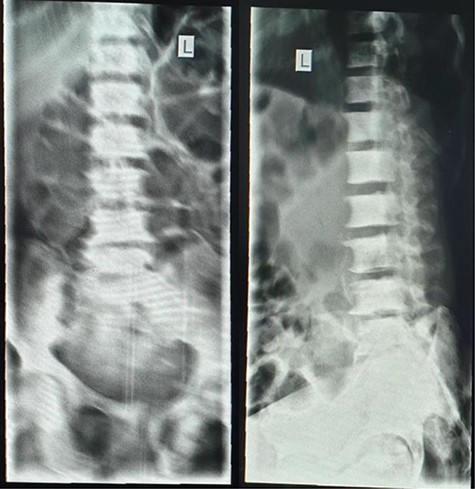
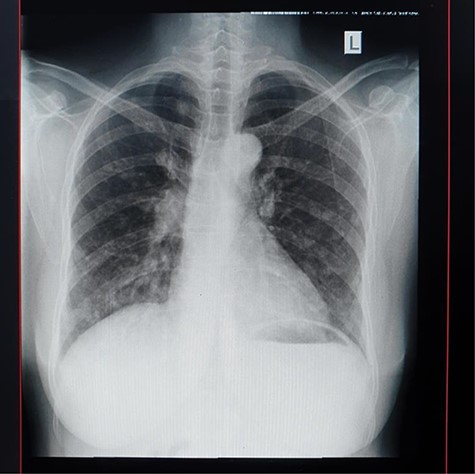
A lumbar sacral X-ray relieved severe osteoblastic changes in the vertebral bodies and the pelvic bones (Fig. 4). On chest X-ray, there were the presence of interstitial changes and increased thoracic cage bone density.
A total of six core biopsies were taken from multiple sites of the prostate. Histopathological examination confirmed the presence of invasive prostatic adenocarcinoma Gleason score 7 (4 + 3) and Schistosoma hematobium eggs (Fig. 5). The patient received a single dose of Praziquantel (40 mg/kg) for the treatment of Schistosomiasis. The patient was referred to oncology department for further management; unfortunately, he got lost to follow-up.
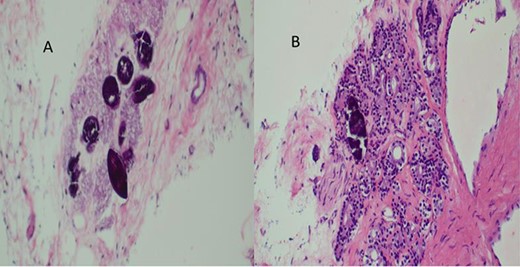
(A) Histopathology of prostatic trucut core biopsy showing infiltrating adenocarcinoma and Schistosoma ova, H&E ×4, ×10 magnification. (B) Prostatic adenocarcinoma and Schistosoma ova, ×40 magnification.
Case number 3
A 53-year-old female presented with recurrent painless gross hematuria for more than 1 year. These symptoms were associated with easy fatigability, heartbeat awareness and weight loss. She has history of working in rice plantation for many years and has had recurrent urinary Schistosomiasis that was treated. However, she denied history of chest pain, change in bowel habits or passing air in urine (pneumaturia). She has been treated in the nearby hospital without any improvement.
On general examination, patient was alert, severely pale, lymphadenopathy, not jaundiced and no pitting lower limb edema. Vitals signs, afebrile, BP 112/70 mmHg, PR 85 B/M, RR 20 B/M.
Per abdomen, there was a mobile, hard suprapubic mass measuring 7 × 7 cm, which was tender on palpation. The liver was normal. Normal bowel sounds were heard. Laboratory findings revealed the Hb level of 6 (13–17 g/dl), creatinine of 1.47 (0.8–1.3 mg/dl), urine culture showed mixed growth. K+ 4.13(3.5–5 mmol/l), Na+ 138.2 (135–145 mmol/l) and Cl+ 100 (95–105 mmol/l). Urinalysis confirmed the presence of too numerous RBC and WBC in urine with bacteria ++.
Radiological findings
An abdominal ultrasound showed a mass in the posterior wall of the urinary bladder measuring 6 cm × 7 cm with areas of necrosis. There was left moderate ureter hydronephrosis. The right kidney and the rest visceral organs were normal. Chest X-ray revealed multiple macronodules all over the lung fields. Heart size, costophrenic and cardiophrenic angles were all normal (Fig. 6).
Urethrocystoscopy found to have extensive sand patch lesion in the urinary bladder with solitary solid tumor on the right side of the bladder wall extending from 3 to 5 o’clock. A transurethral resection of bladder tumor biopsy (TURBx) was done, and histological findings confirmed adenocarcinoma of the bladder with numerous calcified submucosal Schistosoma eggs (Fig. 7).
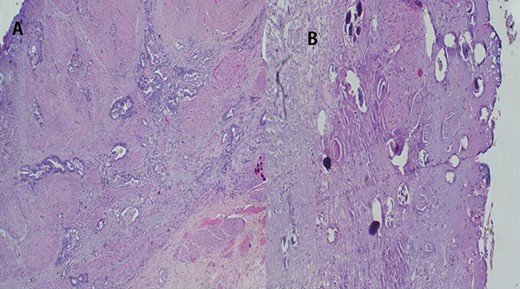
(A) Histopathology of urinary bladder with invasive adenocarcinoma, not otherwise specified (NOS), H&E ×10 magnification. (B) Schistosoma ova near or within the tumor, ×4 magnification.
The final diagnosis of metastatic adenocarcinoma of the urinary bladder was made and the patient was referred for palliative radiation therapy.
DISCUSSION
Cancer is an emerging public health problem in Africa, and infectious agents are the causes of some of the most commonly diagnosed cancers in this region, for instance cancer of cervix, liver, stomach, urinary bladder, Kaposi sarcoma and some lymphomas. A substantial proportion of these cancers are potentially preventable by vaccination, improved hygiene, sanitation and/or treatment. Post Schistosoma infection complications that range from periportal fibrosis with portal hypertension leading to gastrointestinal bleeding and malignancy process are well known [5]. Pathogenesis for neoplasm is induction of chronic inflammatory reaction and irritation leading to the observed consequences in the three cases we have presented [1]. We previously published a case report, from same locality as a patient with colorectal cancer associated with Schistosoma infection [6]. It is a question of what needs to be done to curb these kinds of presentation of the patients in the endemic areas.
Schistosomal colonic disease is a concerning problem in the areas of high prevalence of Schistosomiasis, and if not diagnosed early, it can lead to complications that have high morbidity and mortality. At early stages of the infection, diagnosis is made easily by stool and serum tests for analysis to look for the ova and antigen, respectively. However, the diagnosis of chronic intestinal Schistosomiasis is made by colonic biopsy. Stool and urine analysis is highly specific but has a low sensitivity test value (compared to serum tests); thus, the prevalence is underestimated [7].
The burden of Schistosomiasis is high in sub-Saharan Africa, and it is the second most common NTD [8]. WHO statistics of 2008 indicated that 11.7 million sub-Saharan Africans suffered from Schistosomiasis [8]. In Tanzania, Schistosomiasis is the major under-recognized public health problem. Over 80% of people in endemic areas especially with irrigation and fishing activities are affected [3]. Schistosoma mansoni contributes to the intestinal infection and the latter urogenital Schistosomiasis [3]. Children and young women, especially of child-bearing age, are at most risk of the infection, despite mass control efforts. Common complications from the manifestations are anemia, diarrheal diseases, kidney injury and cancers, including urinary and colorectal cancer [1, 6, 8].
Current measures for treating endemic areas of Tanzania are with Praziquantel by mass deworming treating method in school-aged children, and the aim is to extend the treatment to fishing and agriculture communities and to improve sanitation for preventive measures [3]. Management of the complication following Schistosomal infection poses a great challenge; it imposes a great burden to the already stretched health system. Management of cancer with the needed follow-up brings psychological and financial burden to the patient as well. From the reported prevalence of Schistosomiasis in Tanzania, we anticipate more volume of patients with complications.
A robust screening service that includes drug administration to schools especially in endemic areas to reduce the rates of infection is warranted. This will reduce significantly the prevalence and hence the complications that come with it [9] With regards to neoplasm, a targeted screening services to areas where the disease is endemic may capture patients in pre-clinical states. This may mean conducting random screening endoscopies in selected individuals to assess the mucosal status of the affected organs [9].
As most of the infections occur at around primary school age (7–14 years) [9, 10], screening individuals from 30 years of age in endemic areas with an antibody assay (Schistosoma-specific antibody assay) then subject the positive individuals to endoscopes and random mucosal biopsy can help determine the histological patterns and identify those at risk with pre-malignant lesions or those with dysplasia. The pit fall with this approach is that the mucosal cellular changes depend on the interaction of the Schistosoma eggs and the host immunity, and it is hard to predict when exactly the dysplasia takes place. However, we believe that the random assessment of the affected individuals may facilitate identification and rescheduling surveillance plans.
CONCLUSION
Due to the prevailing prevalence of Schistosomiasis in Tanzania, we therefore recommend an effective control of this infection particularly in endemic areas. This will eventually reduce the oncological complications related to this infection. Also, we recommend epidemiological population studies to bring up the association of chronic Schistosomiasis infection with neoplasm.
ACKNOWLEDGEMENT
The authors would like to thank the patients for giving their consent for their information to be shared for further learning purposes.
Consent
Written informed consent was obtained from the respective patients for publication of this case report and accompanying images. A copy of the consent is available on record.
Authors’ contributions
All authors contributed equally to this manuscript.
Conflict of interest
The authors have no conflict of interests to disclose.



